A 100-mm-long, hollow iron cylinder is exposed to a 1000C carburizing gas (a mixture of CO and
Question:
A 100-mm-long, hollow iron cylinder is exposed to a 1000°C carburizing gas (a mixture of CO and CO2) at its inner and outer surfaces of radii 4.30 and 5.70 mm, respectively. Consider steady-state conditions for which carbon diffuses from the inner surface of the iron wall to the outer surface and the total transport amounts to 3.6 x 10-3 kg of carbon over 100 hours. The variation of the carbon composition (weight % carbon) with radius is tabulated for selected radii.
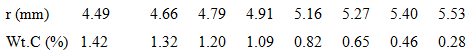
(a) Beginning with Fick's law and the assumption of a constant diffusion coefficient, DC-Fe, show that dPC/d (lnr) is a constant. Sketch the carbon mass density, pC(r), as a function of Inr for such a diffusion process.
(b) The foregoing table corresponds to measured distributions of the carbon mass density. Is DC-Fe a constant for this diffusion process? If not, does DC-Fe increase or decrease with an increasing carbon concentration?
(c) Using the experimental data, calculate and tabulate DC-Fe for selected carbon compositions.
Step by Step Answer:

Fundamentals of Heat and Mass Transfer
ISBN: 978-0471457282
6th Edition
Authors: Incropera, Dewitt, Bergman, Lavine





