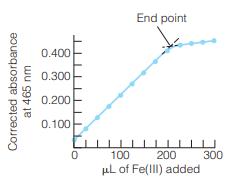
A 2.00-mL solution of apotransferrin was titrated as illustrated in Figure 17-10. It required 163 L of
Question:
A 2.00-mL solution of apotransferrin was titrated as illustrated in Figure 17-10. It required 163 L of 1.43 mM ferric nitrilotriacetateto reach the end point.
(a) Why does the slope of the absorbance-versus-volume graph change abruptly at the equivalence point?
(b) How many moles of Fe(III) (= ferric nitrilotriacetate) were required to reach the end point?
(c) Each apotransferrin molecule binds two ferric ions. Find the concentration of apotransferrin in the 2.00-mL solution.
Figure 17-10
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





