A 25.00-mL volume of commercial hydrogen peroxide solution was diluted to 250.0 mL in a volumetric flask.
Question:
A 25.00-mL volume of commercial hydrogen peroxide solution was diluted to 250.0 mL in a volumetric flask. Then 25.00 mL of the diluted solution were mixed with 200 mL of water and 20 mL of 3 M H2SO4 and titrated with 0.021 23 M KMnO4. The first pink color was observed with 27.66 mL of titrant. A blank prepared from water in place of H2O2 required 0.04 mL to give visible pink color. Using the H2O2 reaction in Table 15-3, find the molarity of the commercial H2O2.
Table 15-3
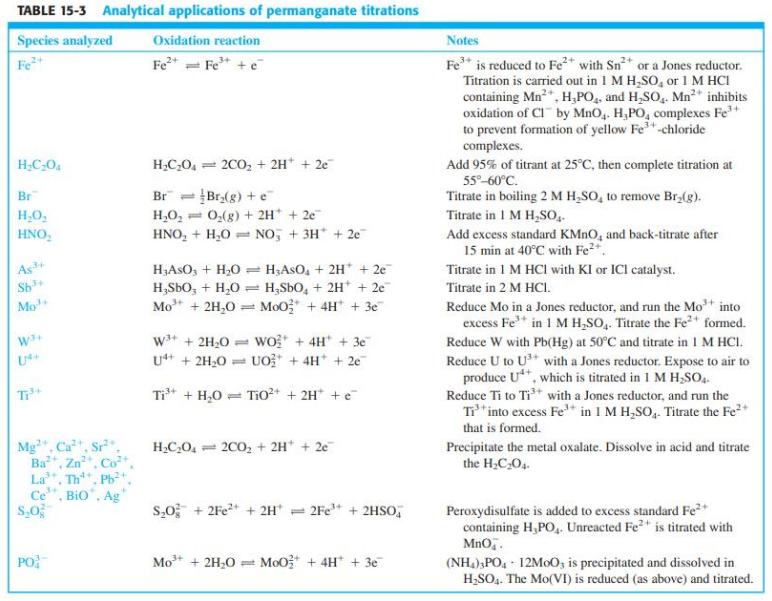
TABLE 15-3 Analytical applications of permanganate titrations Species analyzed Oxidation reaction Notes Fe* = Fe* + e Fe* is reduced to Fe* with Sn2 or a Jones reductor. Titration is carried out in 1 M H,SO, or 1 M HCI containing Mn*, H,PO, and H,SO,. Mn" inhibits oxidation of CI by MnO,. H,PO, complexes Fe to prevent formation of yellow Fe"-chloride complexes. Add 95% of titrant at 25°C, then complete titration at 55°-60°C. Fe H;C-0. H,C0, = 200, + 2H* + 2e Br Br = Br(g) + e Titrate in boiling 2 M H,SO, to remove Br,(g). H,O, = HNO, + H,0 = NO, + 3H* + 2e Titrate in 1 M H,SO, Add excess standard KMNO, and back-titrate after 15 min at 40°C with Fe2*. H,O, 0,(g) + 2H* + 2e HNO, As Sh = HASO, + 2H* + 2e H;SbO, + H,0 = H,SbO, + 2H* + 2e Mo + 2H,0 = MoO; + 4H* + 3e H,AsO, + H;0 Titrate in 1 M HCI with KI or ICl catalyst. Titrate in 2 M HCI. Reduce Mo in a Jones reductor, and run the Mot into excess Fe* in 1 M H,SO,. Titrate the Fe+ formed. Reduce W with Pb(Hg) at 50°C and titrate in I M HCI. Reduce U to U with a Jones reductor. Expose to air to produce U**, which is titrated in 1 M H,So. Reduce Ti to Ti* with a Jones reductor, and run the T*into excess Fe* in 1 M H,SO,. Titrate the Fe* Mo w* + 2H,0 = wo* + 4H + 3e U* + 2H,0 = UO* + 4H* + 2e Ti* + H,0 = Tio* + 2H* +e that is formed. Mg". Ca, Sr Zn La, Th, Ph Ce", Bio", Ag H,C,O, = 2C0, + 2H + 2e Precipitate the metal oxalate. Dissolve in acid and titrate the H;C,0. Ва Peroxydisulfate is added to excess standard Fe* containing H,PO, Unreacted Fe2 is titrated with Mno.. so; + 2Fe* + 2H* = 2Fe* + 2HSO, PO Mo + 2H;O (NH,),PO, - 12M00, is precipitated and dissolved in H,SO. The Mo(VI) is reduced (as above) and titrated. MoO* + 4H* + 3e
Step by Step Answer:
2MnCO 4 5H 2 O 2 6H 2Mn 2 5O 2 8H 2 O 2766004 2762 mL of 0021 23 ...View the full answer



Related Video
Hydrogen peroxide can be used as a mild antiseptic to curb superficial skin infections such as athlete’s foot, but only in diluted quantities. To combat stinky feet, try soaking your feet in a solution of 1 part 3% hydrogen peroxide and 3 parts warm water for 15-20 minutes, then drying them thoroughly. This will kill odor-causing bacteria and soften your feet. To treat athlete\'s foot, you can use a similar solution, but only in diluted quantities, and soak your feet for 30 minutes. Hydrogen peroxide can also be used to keep vegetables fresh by adding 1/4 cup to a bowl of cold water, soaking the vegetables for 20-30 minutes, then draining, drying, and refrigerating them. Alternatively, you can spray vegetables with a solution of 3% hydrogen peroxide and let them stand for a few minutes before rinsing and drying. To keep leftover salad fresh, spray it with a solution of 1/2 cup water and 1 Tbsp. 3% hydrogen peroxide, drain, cover, and refrigerate.
Students also viewed these Chemical Engineering questions
-
The concentration of a hydrogen peroxide solution can be conveniently determined by titration against a standardized potassium permanganate solution in an acidic medium according to the following...
-
Hydrogen peroxide, H2O2, is a colorless liquid. A concentrated solution of it is used as a source of oxygen for rocket propellant fuels. Dilute aqueous solutions are used as a bleach. Analysis of a...
-
Hydrogen peroxide undergoes a first-order decomposition to water and O2 in aqueous solution. The rate constant at 25oC is 7.40 104/s. Calculate the volume of O2 obtained from the decomposition...
-
What does the following code fragment print? String \(s=\) "He11o World"; s. toUpperCase(); s. substring (6, 11); StdOut.println(s);
-
Yahoo! Inc.s recent financial statements contain the following selected data (in thousands). Current assets ...........$ 4,594,772 Total assets ............ 14,936,030 Current liabilities ............
-
A shear layer between two fluids. Assume the following velocity distribution between two shear layers: \[v_{x}(y)=v_{0} \tanh (y / \delta)\] This is known as the Betchov and Criminale form. Calculate...
-
The [+45/-45] laminate described in Problem 7.6 is subjected to a uniaxial force per unit length \(N_{x}=30 \mathrm{MPa} \mathrm{mm}\). Find the resulting stresses and strains in each ply along the...
-
Posa Hotels, Inc., has a sixth night free policy. Every sixth night a guest stays at the hotel is free. Because not all guests stay enough nights to earn a free stay, on average the number of free...
-
Demographics of Toronto ontario Canada (brief description of the city i.e. location, population size of police force, any interesting factors that may contribute to youth crime etc.). What is the...
-
Pro Forma Statements and EFN In the previous question assume Parcells pays out half of net income in the form of a cash dividend. Costs and assets vary with sales, but debt and equity do not. Prepare...
-
When 25.00 mL of unknown were passed through a Jones reductor, molybdate ion (MoO 2- 4 ) was converted into Mo 3+ . The filtrate required 16.43 mL of 0.010 33 M KMnO 4 to reach the purple end point....
-
Two possible reactions of MnO 4 with H 2 O 2 to produce O 2 and Mn 2 are (a) Complete the half-reactions for both schemes by adding e - , H 2 O, and H - and write a balanced net equation for each...
-
Rafael SA is comparing two different options. Nathan T currently uses Option 1, with revenues of 65,000 per year, maintenance expenses of 5,000 per year, and operating expenses of 26,000 per year....
-
Explain why groups and teams are key contributors to organizational effectiveness
-
You hold a diversified portfolio consisting of $2,000 investment in each of five different stocks: The first stock has a beta of 0.68, the second stock has beta of 1.56, the third stock has beta of...
-
Evaluate. S (7x617-10x4/3) d dx
-
A paywall is _ _ _ _ _ _ _ _ . All of these A revenue generator for a news organization more common with traditional newspaper sites than television sites A charge by an internet site to view content.
-
You buy a share of stock, write a one-year call option with X = $25, and buy a one-year put option with X= $25. Your net outlay to establish the entire portfolio is $23.60. What must be the risk-free...
-
Reanalyze the example on film content that was presented in this chapter. Notice that the data set is structured with empathy scored on a scale ranging from none (0) to high (10). Sexual content is a...
-
The graph of an equation is given. (a) Find the intercepts. (b) Indicate whether the graph is symmetric with respect to the x-axis, the y-axis, or the origin. -3 6 -6 3 x
-
Why would a reprecipitation be employed in a gravimetric analysis?
-
Explain how the quartz crystal microbalance at the opening of Chapter 2 measures small masses.
-
Explain what is meant by the statement, "Unless the complete history of any sample is known with certainty, the analyst is well advised not to spend his or her time in analyzing it."
-
What is the expected rate of return on a project that requires an investment of $106 today and generates cash inflows of $7, $17 and $122 in each of the next 3 years?
-
A trader opens a new position by writing two put option contracts. Each contract is on 100 shares of Exxon Mobil common stock. The option premium is $6.06, the strike price is $50, and the stock...
-
On a particular day, there were 300 stocks that advanced on the NYSE and 800 that declined. The volume in advancing issues was 1000 and the volume in declining issues was 3000. What is the common...

Study smarter with the SolutionInn App


