A diagram for an open- tube manometer is shown below. If the flask is open to the
Question:
A diagram for an open- tube manometer is shown below.
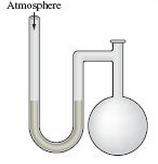
If the flask is open to the atmosphere, the mercury levels are equal. For each of the following situations in which a gas is contained in the flask, calculate the pressure in the flask in torr, atmospheres, and pascals.
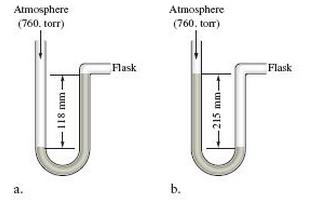
c. Calculate the pressures in the flask in parts a and b (in torr) if the atmospheric pressure is 635 torr.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





