A shell-and-tube heat exchanger in an ammonia plant is preheating 1132 cubic meters of atmospheric pressure nitrogen
Question:
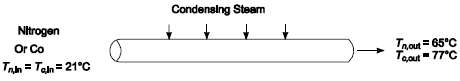
A shell-and-tube heat exchanger in an ammonia plant is preheating 1132 cubic meters of atmospheric pressure nitrogen per hour from 21 to 65?C using steam condensing at 138,000 N/m2. The tube in the heat exchanger have an inside diameter of 2.5 cm. In order to change from ammonia synthesis to methanol synthesis, the same heater is to be used to preheat carbon monoxide from 21 to 77?C, using steam condensing at 241,000 N/m2. Calculate the flow rate which can be anticipated from this heat exchanger in kg of carbon monoxide per second.GIVENShell-and-tube heat exchanger - nitrogen in tubes, condensing steam in shellNitrogen volumetric flow rate (Vn) = 1132 m3/h = 0.3144 m3/sNitrogen temperaturesTn,in = 21?CTn,out = 65?CSteam pressure = 138,000 N/m2Tube inside diameter (Di) = 2.5 cmSame heat exchanger is then used with carbon monoxide:Carbon monoxide temperaturesTc,in = 21?CTc,out = 77?CNew steam pressure = 241 N/m2ASSUMPTIONSTwo or a multiple of two shell passesThermal resistance of the condensing steam and the tube wall are a small fraction of the total thermalresistance
Step by Step Answer:

Principles of heat transfer
ISBN: 978-0495667704
7th Edition
Authors: Frank Kreith, Raj M. Manglik, Mark S. Bohn





