A stream containing H 2 S and inert gases and a second stream of pure SO 2
Question:
A stream containing H2S and inert gases and a second stream of pure SO2 are fed to a sulfur recovery reactor, where the reaction 2H2S + SO2 ? 3S + 2H20 takes place. The feed rates are adjusted so that the ratio of H2S to SO2 in the combined feed is always stoichiometric. In the normal operation of the reactor the flow rate and composition of the H2S feed stream both fluctuate. In the past, each time either variable changed the required SO2 feed rate had to be reset by adjusting a valve in the feed line. A control system has been installed to automate this process. The H2S feed stream passes through an electronic flow meter that transmits a signal R1 directly proportional to the molar flow rate of the stream, h. When n1 = 100 k mol/h, the transmitted signal Rt = 15 mV. The mole fraction of H2S in this stream is measured with a thermal conductivity detector, which transmits a signal Ra. Analyzer calibration data are as follows: The controller takes as input the transmitted values of ft1 and R1 and calculates and transmits a voltage signal R to a flow control valve in the SO2 line, which opens and closes to an extent dependent on the value of Rc. A plot of the SO2 flow rate, h, versus R on rectangular coordinates is a straight line through the points (Rc = 10.0m V, nc = 25.0k mol/h) and (Rc = 25.0m, V nc = 60.0k mol/h).
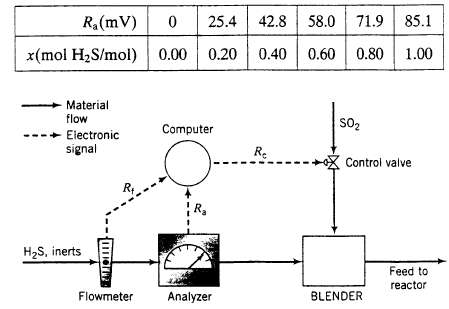
(a) Why would it be important to feed the reactants in stoichiometric proportion? (what are several likely reasons for wanting to automate the SO2 feed rate adjustment?
(b) If the first stream contains 85.0 mole% H2S and enters the unit at a rate of nf = 3.00 x l02 k mol/h, what must the value of nc (k mol S02/h) be?
(c) Fit a function to the H2S analyzer calibration data to derive an expression for x as a function of Ra. Check the fit by plotting both the function and the calibration data on the same graph.
(d) Derive a formula for R( from specified values of Rt and Ra, using the result of part (c) in the derivation. (This formula would be built into the controller.) Test the formula using the flow rate and composition data of part (a).
(e) The system has been installed and made operational, and at some point the concentration of H2S in the feed stream suddenly changes. A sample of the blended gas is collected and analyzed a short time later and the mole ratio of H2S to SO2 is not the required 2:1. List as many possible reasons as you can think of for this apparent failure of the control system.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





