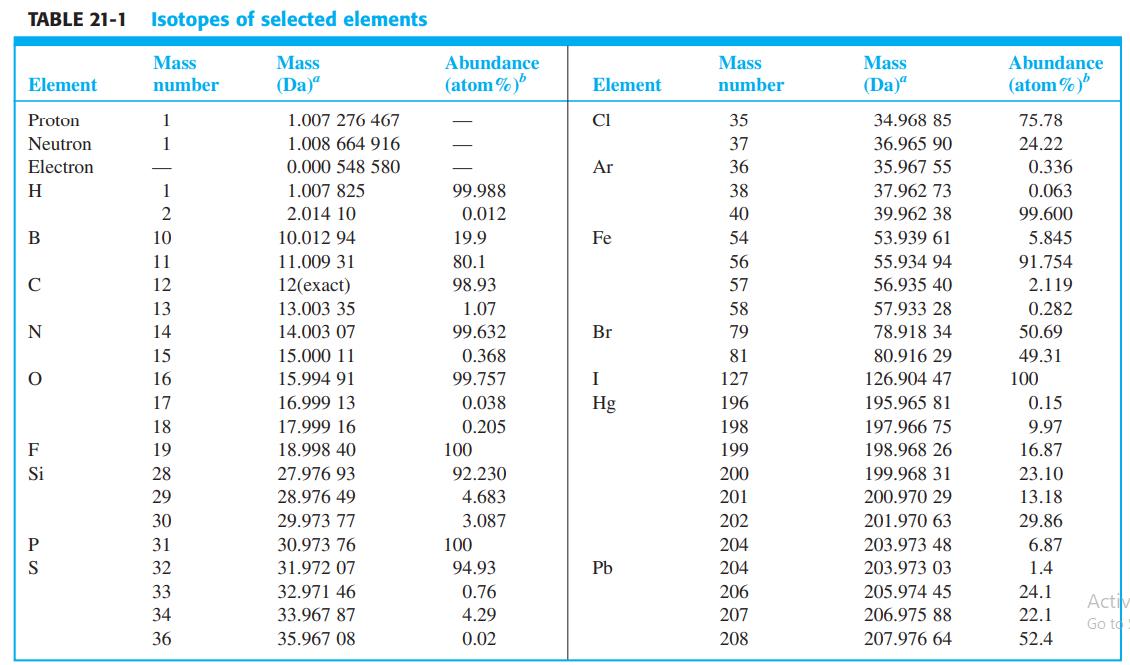
(a) The mass of 1H in Table 21-1 is 1.007 825 Da. Compare it with the sum...
Question:
(a) The mass of 1H in Table 21-1 is 1.007 825 Da. Compare it with the sum of the masses of a proton and an electron given in the table.
(b) 2H (deuterium) contains one proton, one neutron, and one electron. Compare the sum of the masses of these three particles with the mass of 2H.
(c) The discrepancy in part (b) comes from the conversion of mass into binding energy that holds the nucleus together. The relation of mass, m, to energy, E, is E = mc2, where c is the speed of light. From the discrepancy in part (b), calculate the binding energy of 2H in joules and in kJ/mol. (1 Da 1.660 5 × 10-27 kg)
(d) The binding energy (ionization energy) of the electron in a hydrogen or deuterium atom is 13.6 eV. Use Table 1 - 4 to convert this number into kJ/mol and compare it with the binding energy of the 2H nucleus.
(e) A typical bond dissociation energy in a molecule is 400 kJ/mol. How many times larger is the nuclear binding energy of 2H than a bond energy?
Table 21-1
Step by Step Answer:






