A vapor mixture having equal volumes of NH3 and N2 is to be contacted at 20?C and
Question:
A vapor mixture having equal volumes of NH3 and N2 is to be contacted at 20?C and 1 atm (760 torr) with water to absorb a portion of the NH3. If 14 m3 of this mixture is brought into contact with 10 m3 of water and if equilibrium is attained, calculate the percent of the ammonia originally in the gas that will be absorbed. Both temperature and total pressure will be maintained constant during the absorption. The partial pressure of NH3 over water at 20?C is as follows:
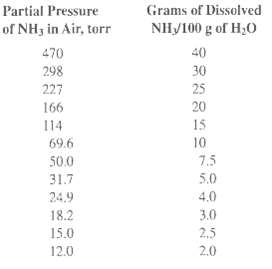
Transcribed Image Text:
Partial Pressure Grams of Dissolved of NH3 in Air, torr NH/100 g of H;0 470 40 30 298 25 227 20 166 15 114 10 69.6 7.5 50.0 31.7 5.0 4.0 24.9 18.2 3.0 2,5 15.0 2.0 12.0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
First compute the kmol of water Density 1000 kgm 3 M of water 1802 Therefore we have 1010001802 555 ...View the full answer

Answered By

Deepak Pal
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students. Areas of interest: Business, accounting, Project management, sociology, technology, computers, English, linguistics, media, philosophy, political science, statistics, data science, Excel, psychology, art, history, health education, gender studies, cultural studies, ethics, religion. I am also decent with math(s) & Programming. If you have a project you think I can take on, please feel welcome to invite me, and I'm going to check it out!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
A quantity of N2 gas originally held at 5.25 atm pressure in a 1.00-L container at 26oC is transferred to a 12.5-L container at 20oC .A quantity of O2 gas originally at 5.25 atm and 26oC in a 5.00-L...
-
Ammonia (NH 3 ) in air is being absorbed into water within the enclosed tank shown in the figure (next page). The liquid and gas phases are both well mixed, and mass-transfer occurs only at the...
-
A mixture of N2, H2 and NH3 is at equilibrium according to the equation N2(g) + 3H2(g) 2NH3(g) as depicted below. The volume is suddenly decreased (by increasing the external pressure), and a new...
-
What are the premises for successful paleostress analysis?
-
At April 30, the bank reconciliation of Frobisher shows three outstanding cheques: No. 254, $1,120; No. 255, $1,600; and No. 257, $820. A list of cheques recorded by the bank and the company in the...
-
The following income statement does not reflect intraperiod tax allocation. Required: Recast the income statement to reflect intraperiod tax allocation. INCOME STATEMENT For the Fiscal Year Ended...
-
The hangers support the joist in such a way that the four nails on each hanger can be assumed to support an equal portion of the load. If the joist is subjected to the loading shown, determine the...
-
Rene Rogers has adjusted gross income of $40,000. She has medical expenses of $5,000 and $3,000 of miscellaneous itemized deductions subject to the two-percent-of-AGI limit. In addition, she has a...
-
The Offline Impact of Online Ads-The Harvard Business Review article-https://services.hbsp.harvard.edu/api/courses/1087075/items/F0804H-PDF-ENG/sclinks/5f6f170df4f4c8c9f395f8a4f24d243a What is the...
-
New Horizons Co. is a high-tech firm whose owner does not have the required management expertise to run the firm. The owner wants to hire a manager with the required expertise. The continued success...
-
It has been proposed that oxygen be separated from nitrogen by absorbing and desorbing air in water. Pressures from 101.3 to 10,130 kPa and temperatures between 0 and 100C are to be used. (a) Devise...
-
Repeat Example 4.15 for temperatures corresponding to the following vapor pressures for solid PA: (a) 0.7 torr (b) 0.4 torr (c) 0.1 torr Plot the percent recovery of PA versus the solid vapor...
-
Given the block diagram, find the system's I/O equation. U(s) FIGURE 4.43 Problem 14. -IN 11 4 15 15 S 12 -- 2 1 4 Y(s)
-
What is the output of the following? A. sugar sugar 3 B. sugar minnie 1 C. snowball minnie 1 D. snowball snowball 3 E. The code does not compile. F. None of the above. var q = new ArrayDeque ();...
-
Given the following class diagram, which two classes are missing in the hierarchy at positions 1 and 2? A. IOException at position 1, Exception at position 2 B. Exception at position 1,...
-
What is the output of the following? (Choose two.) A. 1843 B. 1922 C. 1974 D. The code compiles but throws an exception at runtime. 35: var mags new HashMap (); 36: mags.put("People", 1974); 37:...
-
What is the output of the following? A. [Natural History, Science] B. [Natural History, Art, Science] C. The code does not compile. D. The code compiles but throws an exception at runtime. List...
-
Which of the following cannot fill in the blank to make the code compile? A. Collection B. LinkedList C. TreeMap D. None of these can fill in the blank. E. All of these can fill in the blank. private...
-
Describe the two main classifications of interests in land. Distinguish between freehold and leasehold estates.
-
If the jobs displayed in Table 18.24 are processed using the earliestdue-date rule, what would be the lateness of job C? TABLE 18.24 Processing Times and Due Dates for Five Jobs Job C D E...
-
What is the difference between the functions of employee representatives and employer organizations?
-
What are the four idealized flow patterns in membrane modules? Which is the most effective? Which is the most difficult to calculate?
-
How do the solution-diffusion equations differ for liquid transport and gas transport? How is Henrys law used for solution-diffusion for gas transport? Why are the film resistances to mass transfer...
-
Water at 70C is passed through a polyethylene membrane of 25% porosity with an average pore diameter of 0.3 m and an average tortuosity of 1.3. The pressures on the downstream and upstream sides of...
-
What is the expected rate of return on a project that requires an investment of $106 today and generates cash inflows of $7, $17 and $122 in each of the next 3 years?
-
A trader opens a new position by writing two put option contracts. Each contract is on 100 shares of Exxon Mobil common stock. The option premium is $6.06, the strike price is $50, and the stock...
-
On a particular day, there were 300 stocks that advanced on the NYSE and 800 that declined. The volume in advancing issues was 1000 and the volume in declining issues was 3000. What is the common...

Study smarter with the SolutionInn App


