Ammonia is one of the chemical constituents of industrial waste that must be removed in a treatment
Question:
Ammonia is one of the chemical constituents of industrial waste that must be removed in a treatment plant before the waste can safely be discharged into a river or estuary. Ammonia is normally present in wastewater as aqueous ammonium hydroxide (NH+4OH???). A two-part process is frequently carried out to accomplish the removal. Lime (CaO) is first added to the wastewater, leading to reaction the hydroxide ions produced in this reaction drive the following reaction to the right, resulting in the conversion of ammonium ions to dissolved ammonia: Air is then contacted with the wastewater stripping out the ammonia.
(a) One million gallons per day of alkaline wastewater containing 0.03 mole NH3/mole ammonia- free H2O is fed to a stripping tower that operates at 68°F. Air at 68°F and 21.3psia contacts the wastewater counter currently as it passes through the tower. The feed ratio is 300ft3air/gal wastewater, and 93% of the ammonia is stripped from the wastewater. Calculate the volumetric flow rate of the gas leaving the tower and the partial pressure of ammonia in thisgas.
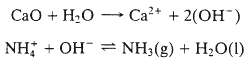
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





