Benzoic acid is to be crystallized by bulk-phase desublimation from N2 using a novel method described by
Question:
Benzoic acid is to be crystallized by bulk-phase desublimation from N2 using a novel method described by Vitovec, Smolik, and Kugler [Coll. Czech. Chem. Commun., 42, 1108-1117 (1977)]. The gas, containing 6.4 mol% benzoic acid and the balance N2, flows at 3 m3/h at 1 atm and a temperature of 10?C above the dew point. The gas is directly cooled by the vaporization of 150 cm3/h of a water spray at 25?C. The gas is further cooled in two steps by nitrogen quench gas at 1 atm as follows:
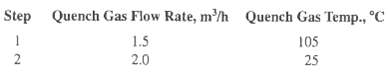
The quench gases enter through porous walls of the vessel so as to prevent crystallization on the vessel wall. Based on the following data for benzoic acid, determine the final gas temperature and the fractional yield of benzoic-acid crystals, assuming equilibrium in the exiting gas.
Melting point = 122.4oC
Specific heat of solid and vapor = 0.32 cal/g-oC
Heat of sublimation = 134 cal/g
Vapor pressure:
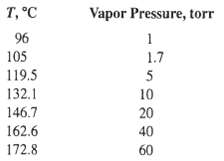
The vapor pressure data can be extrapolated to lower temperatures by the Antoine equation.
Step by Step Answer:






