Calculate the pressure exerted by 0.5000 mole of N 2 in a 1.0000-L container at 25.0 o
Question:
Calculate the pressure exerted by 0.5000 mole of N2 in a 1.0000-L container at 25.0oC. (See Table)
a. Use the ideal gas law.
b. Use the van der Waals equation.
c. Compare the results from parts a and b.
Table
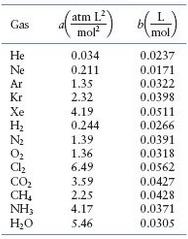
Transcribed Image Text:
\ 7 1 200 1 6 1 8 2 7815 2133 (000000000000 691622 23354433 LF- 11 5 2 9 4 9699576 345214 m | 0 2 3 3 1 2 3 0012401163245
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 37% (8 reviews)
a b c PV nRT P n...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physical Chemistry questions
-
Calculate the pressure exerted by 1.0 mol H2S behaving as (a) A perfect gas, (b) A van der Waals gas when it is confined under the following conditions: (i) At 273.15 K in 22.414 dm3, (ii) At 500 Kin...
-
Van der Waals Equation and Critical Points (a) In p V- diagrams the slope p/V along an isotherm is never positive. Explain why. (b) Regions where p/V = 0 represent equilibrium between two phases;...
-
By what magnitude would the pressure exerted by water on the walls of the vessel have increased if the intermolecular attraction forces had vanished?
-
Assume that your team has been in contract with the headquarters of a company that owns several restaurants in different states in the US. Your team is to provide software that manages these...
-
Allies Apples, Inc. purchases apples in bulk and sells two products, boxes of apples and jugs of cider. Allies has capacity limitations of three kinds: warehouse space, crating facilities, and...
-
Obtain the transfer function X(s)/F(s) from the block diagram shown in Figure. G(s) Fs) + X(s)
-
Beth worked as a lifeguard for several summers while attending college. During the course of her employment, she and other female lifeguards were subject to uninvited and offensive touching by their...
-
A firm has the following items on its balance sheet: Cash ........................$ 20,000,000 Inventory ...................... 134,000,000 Notes payable to bank ................ 31,500,000 Common...
-
How will the Information Assurance program benefit you in the future?
-
Murray Hill was preparing the monthly report that allocates the three service departments ( A, B, and C) costs to the three operating divisions ( D1, D2, and D3) when he choked to death on a stale...
-
It took 4.5 minutes for 1.0 L of helium to effuse through a porous barrier. How long will it take for 1.0 L of Cl 2 gas to effuse under identical conditions?
-
Calculate the pressure exerted by 0.5000 mole of N 2 in a 10.000- L container at 25.0 o C. (See Table) a. Use the ideal gas law. b. Use the van der Waals equation. c. Compare the results from parts a...
-
Graph each parabola. Give the vertex, axis of symmetry, domain, and range. f(x) = x + 2
-
need help resolving this on excel please 1 The Company WSpectacles is a firm that organizes concerts, festivals and spectacles in Paris. In particular, WSpectacles focuses on events for young people,...
-
Who is a great example of a Transactional Leadership and Laissez-Faire Leadership in the 20 century?
-
How might the consequences of the situation have been different if acted as a Creator?
-
About costs incurred after the acquisition, what does increased service potential mean?
-
Define the significant differences and similarities between empowerment and laissez-faire leadership.?
-
Show that the commutation relation for the electron spatial field operators is using the normalization relation for the Bloch cell functions {G), (Gr')} = 8&r 7')n,n', - n (4.6.6)
-
Distinguish among total-moisture content, free-moisture content, equilibrium-moisture content, unbound moisture, and bound moisture.
-
A certain oxide of titanium is 28.31% oxygen by mass and contains a mixture of Ti2+ and Ti3+ ions. Determine the formula of the compound and the relative numbers of Ti2+ and Ti3+ ions.
-
Spinel is a mineral that contains 37.9% aluminum, 17.1% magnesium, and 45.0% oxygen, by mass, and has a density of 3.57 g/cm3. The edge of the cubic unit cell measures 809 pm. How many of each type...
-
A metallic solid with atoms in a face-centered cubic unit cell with an edge length of 392 pm has a density of 21.45 g/cm3. Calculate the atomic mass and the atomic radius of the metal. Identify the...
-
According to the Hawail Wildlife Fund, North Pacific Humpback Whales migrate from the icy waters around Alaska during the fall to spend the winter in Hawaii where they mate, give birth, and nurture...
-
Find the limit 4 lim (x -2x32x + x 1) =? 2+1 -
-
Train-the-trainer manual for the training and development department within your organization or for one with which you are familiar. Your trainers will soon be responsible to train the staff about...

Study smarter with the SolutionInn App


