Ca(OH) 2 has a K sp of 6.5 X 10 -6 (a) If 0.370 g of Ca(OH)
Question:
Ca(OH)2 has a Ksp of 6.5 X 10-6
(a) If 0.370 g of Ca(OH)2 is added to 500 mL of water and the mixture is allowed to come to equilibrium,will the solution be saturated?
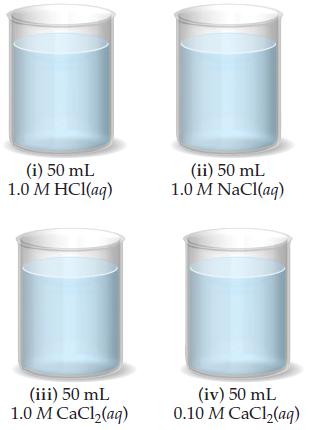
(b) If 50 mL of the solution from part (a) is added to each of the beakers shown here, in which beakers, if any,will a precipitate form? In those cases where a precipitate forms, what is its identity?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0321696724
12th edition
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
Question Posted:





