Consider a gas of diatomic molecules (moment of inertia I) at an absolute temperature T. If Eg
Question:
Consider a gas of diatomic molecules (moment of inertia I) at an absolute temperature T. If Eg is a ground-state energy and E ex is the energy of an excited state, then the Maxwell-Boltzmann distribution (see Section 38.6) predicts that the ratio of the numbers of molecules in the two states is(a) Explain why the ratio of the number of molecules in the l th rotational energy level to the number of molecules in the ground (l = 0) rotational level is(b) Determine the ratio n1/n0 for a gas of CO molecules at 300 K for the cases (i) l = 1; (ii) l = 2; (iii) l = 10;(iv) l = 20; (v) l = 50. The moment of inertia of the CO molecule is given in Example 42.2 (Section 42.2).(c) Your results in part (b) show that as I is increased, the ratio n1/n2 first increases and then decreases. Explainwhy.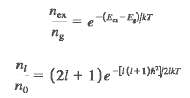
The word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Step by Step Answer:






