Consider the titration in Figure 15-2. (a) Write a balanced titration reaction. (b) Write two different half-reactions
Question:
Consider the titration in Figure 15-2.
(a) Write a balanced titration reaction.
(b) Write two different half-reactions for the indicator electrode.
(c) Write two different Nernst equations for the cell voltage.
(d) Calculate E at the following volumes of Ce4+: 10.0, 25.0, 49.0, 50.0, 51.0, 60.0, and 100.0 mL. Compare your results with Figure 15-2.
Figure 15-2
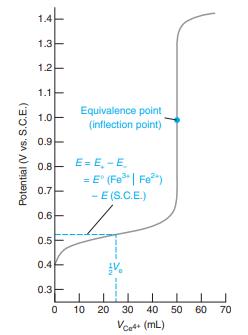
Transcribed Image Text:
1.4 1.3 1.2 1.1 Equivalence point (inflection point) 1.0 0.9 0.8- E= E, -E = E" (Fe| Fe") - E (S.C.E.) 0.7 0.6- 0.5 0.4 0.3 10 20 30 40 50 60 70 Vcet+ (mL) Potential (V vs. S.C.E.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
a b c d Ce4 Fe2 Ce Fe Fe3 efe2 Eo 0767 V Ce4eCe3 E 170 V E 07670059 16 log 100 mL E ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Consider the titration of 50.0 mL of 1.0 M glycine hy-drochloride [(H3NCH2COOH)CI], with 1.0 M NaOH. For +H3NCH2COOH, Ka for the carboxylic acid group is 4.3 X 10-3 and Kb, for the amino group is 6.0...
-
Consider the titration of a generic weak acid HA with a strong base that gives the following titration curve: On the curve indicate the points that correspond to the following. a. the equivalence...
-
Consider the titration of 100.0 mL of 0.10 M phosphoric acid with 0.10 M NaOH. a. Determine the pH at the third half- equivalence point by assuming it is a special point (see Fig.). b. Calculate the...
-
Draw the BST that results when you insert the keys E A S Y QUE S T I O N in that order into an initially empty tree. What is the height of the resulting BST?
-
Swenson Company has the following payroll procedures. (a) Supervisor approves overtime work. (b) The human resources department prepares hiring authorization forms for new hires. (c) A second payroll...
-
Search the literature or web and discuss briefly the principles behind the following flowmeasurement devices: a Pitot tube; a hot-wire anemometer; a laser-Doppler velocity meter; and a...
-
An antisymmetric angle-ply \([+\theta /-\theta]\) laminate is to be made of carbon/ epoxy and designed to have a laminate CTE, \(\alpha_{x}\) as close to zero as possible. Determine the ply...
-
Refer to Appendix 2 and calculate the incremental contribution realized by adding the new iPhone 4 if sales during the first six months of launch were five million units. However, the company also...
-
Give your opinion/response to the below- Lukis Anderson arrested December 2012, for murder of Raveesh Kumra but actually it was more of framed by his own DNA. His DNA was found under the finger nail...
-
"A producer of computer aided design software for the aerospace industry receives numerous calls for technical support. Tracking software is used to monitor response and resolution times. In...
-
A solution containing 50.0 mL of 0.100 M EDTA buffered to pH 10.00 was titrated with 50.0 mL of 0.020 0 M Hg(ClO 4 ) 2 in the cell shown in Exercise 14-B: S.C.E. 7 titration solution | Hg(l) From the...
-
Why don't Cr 3+ and TiO 2+ interfere in the analysis of Fe 3 + when a Walden reductor, instead of a Jones reductor, is used for prereduction?
-
Find each derivative at the given x-value (a) With the appropriate rule (b) With the numerical derivative feature of a graphing calculator. 1. y = 5 - 2 x at x = 4 2. y = 1 + 3x2/3 at x = - 8
-
Which one of the following is normally not included in a product specification? a. the item's intended use b. Edible Price c. packer's brand name d. package size
-
How the International Standard can assist with the development and application of risk management plans and strategies ?
-
How does the HR function relate to the strategy of its organization? Describe how HR can use metrics to measure the effectiveness of HR from both a strategic and operational perspective. Why must...
-
Do you believe that a good structure can enhance organizational effectiveness? give a brief example
-
Solve t/me sin 2x - dx. cos 2x 0
-
A computer company conducted a new and improved course designed to train its service representatives in learning to repair personal computers. Twenty trainees were split into two groups on a random...
-
If |62x|>9, which of the following is a possible value of x? A. 2 B. 1 C. 0 D. 4 E. 7
-
A method to measure soluble organic carbon in seawater includes oxidation of the organic materials to CO2 with K2S2O8, followed by gravimetric determination of the CO2 trapped by a column of...
-
How many milliliters of 2.15% alcoholic dimethylglyoxime should be used to provide a 50.0% excess for Reaction 26-7 with 0.9984g of steel containing 2.07 wt% Ni? Assume that the density of the...
-
Twenty dietary iron tablets with a total mass of 22.131 g were ground and mixed thoroughly. Then 2.998 g of the powder were dissolved in HNO3 and heated to convert all iron into Fe3+. Addition of NH3...
-
x-8x+15 A) Let f(x) = = x2+2x-15 Calculate lim f(x) x 3 2x225x75 B) Let f(x) = x-18x45 Calculate lim f(x) x 15
-
If a dance instructor prices her lessons at $60 per student, she will have three students. If she prices her lessons at $50 per student, she will have four students. How much marginal revenue will...
-
What is the difference between objectives, strategies, and goals? How do these things improve our planning process?

Study smarter with the SolutionInn App


