(Each part of this problem is quite long and best worked by groups of students.) Peak intensities...
Question:
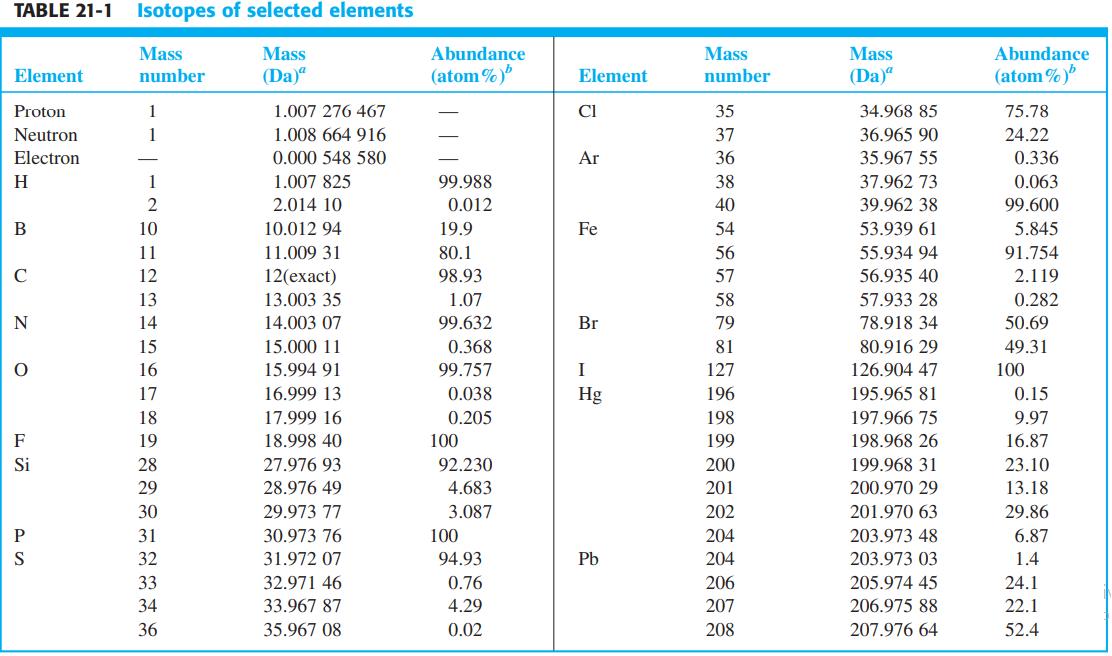
(Each part of this problem is quite long and best worked by groups of students.) Peak intensities of the molecular ion region are listed in parts (a) - (g) and shown in the figure. Identify which peak represents the molecular ion, suggest a composition for it, and calculate the expected isotopic peak intensities. Restrict your attention to elements in Table 21-1.
(a) m/z (intensity): 112 (999), 113 (69), 114 (329), 115 (21)
(b) m/z (intensity): 146 (999), 147 (56), 148 (624), 149 (33), 150 (99), 151 (5)
(c) m/z (intensity): 90 (2), 91 (13), 92 (96), 93 (999), 94 (71), 95 (2)
(d) m/z (intensity): 226 (4), 227 (6), 228 (130), 229 (215), 230 (291), 231 (168), 232 (366), 233 (2), 234 (83) (Calculate expected intensities from isotopes of the major element present.)
(e) m/z (intensity): 172 (531), 173 (12), 174 (999), 175 (10), 176 (497)
(f) m/z (intensity): 177 (3), 178 (9), 179 (422), 180 (999), 181 (138), 182 (9)
(g) m/z (intensity): 182 (4), 183 (1), 184 (83), 185 (16), 186 (999), 187 (132), 188 (10)
.png)
Table 21-1
Step by Step Answer:






