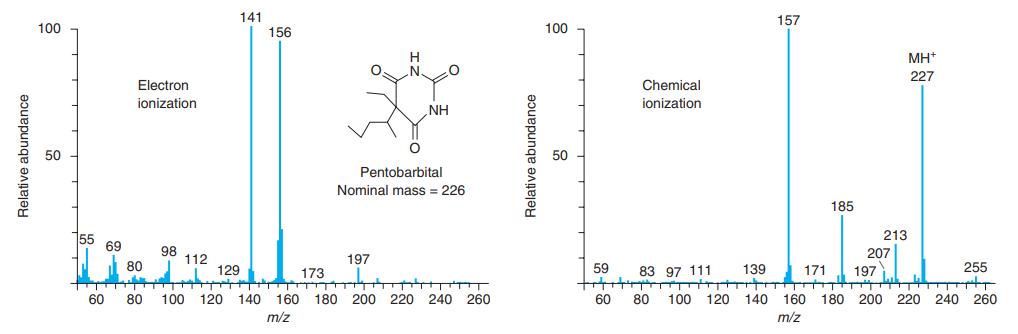
How are ions created for each of the mass spectra in Figure 21-4? Why are the two
Question:
How are ions created for each of the mass spectra in Figure 21-4? Why are the two spectra so different?
Figure 21-4
Transcribed Image Text:
141 156 157 100 100 H .N. MH* 227 Electron Chemical ionization ionization NH 50 50 Pentobarbital Nominal mass = 226 185 155 69 213 98 112 197 207 80 59 255 171 +tt t T 140 129 173 83 97 111 139 197 60 80 100 120 140 160 180 200 220 240 260 60 80 100 120 160 180 200 220 240 260 m/z m/z Relative abundance Relative abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
For the electron impact spectrum pentobarbital is bomba...View the full answer

Answered By

Milan Mondal
I am milan mondal have done my Msc in physics (special astrophysics and relativity) from the University of burdwan and Bed in physical science from the same University.
From 2018 I am working as pgt physics teacher in kendriya vidyalaya no2 kharagpur ,west bengal. And also I am doing advanced physics expert in chegg.com .also I teach Bsc physics .
I love to teach physics and acience.
If you give me a chance I will give my best to you.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
For each of the following ions, indicate the total number of protons and electrons in the ion. For the positive ions in the list, predict the formula of the simplest compound formed between each...
-
When a metal ion has a coordination number of 2, 4, or 6, what are the observed geometries and associated bond angles? For each of the following, give the correct formulas for the complex ions. a....
-
For each of the following pairs of ions, write the formula of the corresponding compound. a. Co2+ and N3 b. NH4+ and PO43 c. Na+ and CO32 d. Fe3+ and OH
-
What are the important types of financial intermediaries in the U.S. economy? What are the primary assets of these intermediaries, and how do they facilitate investment spending and saving?
-
Presented below is information related to Gantner Company for its first month of operations. Identify the balances that appear in the accounts receivable subsidiary ledger and the accounts receivable...
-
Nuts and Cholesterol Several studies have been performed to examine the relationship between nut consumption and cholesterol levels. Here we consider two such studies. In Study 1, participants were...
-
True or False: A rotor that has been balanced down to the G specification is considered ready to ship.
-
In the course of producing its output, a firm causes pollution. The government passes a law that requires the firm to stop polluting, and the firm discovers that it can prevent the pollution by...
-
A $980m hedge fund charges fees of 1.5-20 and has 0.22% in other annual expenses. LPs provide $945m of the initial capital with the GP providing the rest. The GP takes the management fees out of the...
-
The homogeneous wire ABC is bent into a semicircular arc and a straight section as shown and is attached to a hinge at A. Determine the value of ( for which the wire is in equilibrium for the...
-
Explain how a double-focusing mass spectrometer achieves high resolution.
-
A limitation on how many spectra per second can be recorded by a time-of-flight mass spectrometer is the time it takes the slowest ions to go from the source to the detector. Suppose we want to scan...
-
Derive the following Maxwell relations for open systems. a. Starting from Eq. 6.2-5a, b. Starting from Eq. 6.2-6a, c. Starting from Eq. 6.2-7a, d. Starting from Eq. 6.2-8a, (). HT ON (0) aN S, N S,V...
-
The list of 3 jobs Ross Co. worked on during the year: Cost Job B1 Job O1 Job B2 Prime costs $4,100 $6,250 $7,400 Manufacturing overhead ? ? ? Bob applies overhead at a rate of 65% of prime costs....
-
The client is employed in Amazon, India, and receives a competitive annual package that includes ESOPs that have increased in value. With a taste for success, your client is investing a large amount...
-
Write a C program named as countD.c that takes input text file and a digit as arguments, outputs how many times the digit appears in the file. If the text file does not exist, print out an error...
-
Economic progress within a country seems to depend on how well property rights are protected. Explain the relationship between property rights, corruption, and economic progress and their importance...
-
Discuss the characteristics of the rule of law, according to Hayek.
-
A judge in Lamar County, Texas, ruled that TransCanada has permission to build its Keystone XL pipeline from Cushing, Oklahoma, to Port Arthur, Texas. TransCanada has said it will start building as...
-
-4 1 9. Let A = Find A-1, (A") and verify that (A")= (A-1)".
-
The purity of cocaine bought on the street varies dramatically. Cutting agents include levamisole, a compound normally used to kill parasites. Search the literature for a reversed-phase liquid...
-
Human serum albumin (HSA) is an important protein ingredient in cryopreservation media used in procedures such as in vitro fertilization. Search the literature for a highperformance liquid...
-
State the effects of increasing cross-linking on an ion exchange column.
-
Why is compassion and empathy valuable traits to have as a leader ?
-
what leadership development activities are most likely to be successful for the TRS society
-
As a support services worker, you may be tasked with not only assisting your assigned client but also the members of the community in which your client will reside. You will also be a part of case...

Study smarter with the SolutionInn App


