Hydrogen is produced in the steam reforming of propane: C 3 H 8 (g) + 3 H
Question:
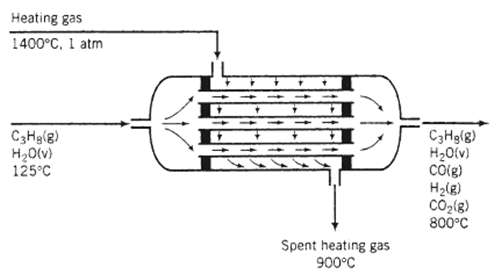
Hydrogen is produced in the steam reforming of propane: C3H8 (g) + 3 H2O (v) ? 3 CO2 (g) + 7 H2 (g) the water?gas shift reaction also takes place in the reactor, leading to the formation of additional hydrogen: CO (g) + H2O (v) ? CO2 (g) + H2 (g) the reaction is carried out over a nickel catalyst in the tubes of a shell-and-tube reactor. The feed to the reactor contains steam and propane in a 6:1 molar ratio at 125?C, and the products emerge at 800?C. The excess steam in the feed assures essentially complete consumption of the propane. Heat is added to the reaction mixture by passing a hot gas over the outside of the tubes that contain the catalyst. The gas is fed at 4.94 m3/mol C3H8 entering the unit at 1400?C and 1 atm and leaving at 900?C. The unit may be considered adiabatic. Calculate the molar composition of the product gas assuming that the heat capacity of the heating gas is 0.040 kJ/ (mol??C).
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





