In the preliminary design of a furnace for industrial boiler, methane at 25?C is burned completely with
Question:
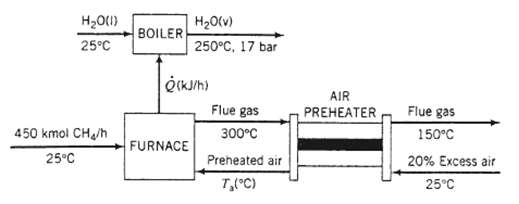
In the preliminary design of a furnace for industrial boiler, methane at 25?C is burned completely with 20% excess air, also at 25?C. The feed rate of methane is 450k mol/h. The hot combustion gases leave the furnace at 300?C and are discharged to the atmosphere. The heat transferred from the furnace (Q) is used to convert boiler feed water at 25?C into super heated steam at 17 bar and 250?C. Use the following approximate heat capacities [kJ/ (mol??C)] in your solution of this problem: CH4 (g) = 0.0431, CO2 (g) = 0.0423, H2O (g) = 0.0343, O2 (g) = 0.0312, N2 (g) 0.0297.
(a) Draw and label a flowchart of this process [the chart should look like the one shown in part (b) without the pre-heater and calculate the composition of the gas leaving the furnace. Then, using the given constant heat capacities, calculate the average molar heat capacity of the gas. (See Equation 8.3-13.) Finally, calculate Q(kJ/h) and the rate of steam production in the boiler (kWh).
(b) In the actual boiler design the air feed at 25?C and the combustion gas leaving the furnace at 300?C pass through a heat exchanger (the air pre-heater). The combustion (flue) gas is cooled to 150?C in the pre-heater and is then discharged to the atmosphere, and the heated air is fed to the Calculate the temperature of the air entering the furnace (a trial-and-error solution is required) and the rate of steam production (kg/h).
(c) Explain why preheating the air increases the rate of steam production. (Suggestion: Use the energy balance on the furnace in your explanation.) Why does it make sense economically to use the combustion gas as the heating medium?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





