Kevlar (C 14 H 10 N 2 O 2 ) is used in various applications from tires
Question:
Kevlar (C14H10N2O2) is used in various applications from tires to body armor due to its high strength-to-weight ratio. The polymer is produced from the monomers paraphenylene diamine (C6H8N2) and terephthaloyl (C8H4Cl2O2) by the following reaction:
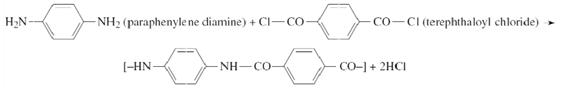
(a) What type of polymerization does the above reaction represent?
(b) Assuming 100% efficiency, calculate the weight of terephthaloyl chloride required to completely combine with 1 kg of paraphenylene diamine.
(c) How much Kevlar is produced?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Essentials of Materials Science and Engineering
ISBN: 978-1111576851
3rd edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:





