Nitric acid is produced commercially by the Ostwald process. In the first step, ammonia is oxidized to
Question:
Nitric acid is produced commercially by the Ostwald process. In the first step, ammonia is oxidized to nitric oxide:
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
Assume this reaction is carried out in the apparatus diagramed below.
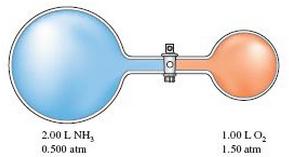
The stopcock between the two reaction containers is opened, and the reaction proceeds using proper catalysts. Calculate the partial pressure of NO after the reaction is complete. Assume 100% yield for the reaction, assume the final container volume is 3.00 L, and assume the temperature is constant.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





