Nitric acid is used extensively for the production of inorganic and organic nitrates, for metal treatments of
Question:
Nitric acid is used extensively for the production of inorganic and organic nitrates, for metal treatments of various kinds, and for photoengraving. It is produced by oxidizing ammonia to nitric oxide over a platinum?rhodium catalyst, oxidizing the nitric oxide to nitrogen dioxide, and dissolving the NO2 in water:
4 NH3 (g) + 5 O2 (g) ? 4 NO (g) + 6 H2O (g)
2 NO (g) + O2 (g) 2 NO2 (g)
3 NO2 (g) + H2O (l) ? 2 HNO3 (aq) + NO (g)
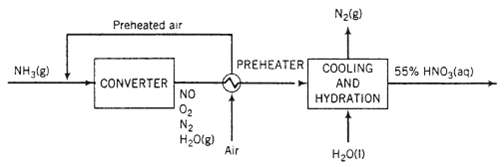
A side reaction that lowers the product yield is the oxidation of ammonia to nitrogen and water vapor: 4 NH3 (g) + 3 O2 (g) ?2 N2 (g) ? 6 H2O (g) Saturated ammonia vapor, produced by vaporizing pure Liquid ammonia at 820 kPa absolute, is mixed with a stoichiometric quantity of air, and the combined stream enters a converter. Prior to being mixed with the ammonia, the air is compressed and passed through a pre-heater, it enters the compressor at 30?C and 1 atm with a relative humidity of 50%, and it exchanges heat in the pre-heater with the gases emerging from the converter. The quantity of oxygen in the feed is the amount theoretically required to convert all of the ammonia to HNO3. In the converter, the ammonia reacts completely, with 97% forming NO and the balance forming N2. In the short time in which the reaction mixture is in the presence of the catalyst (less than 0.001 s), a negligible amount of NO2 is formed. The product gas is subjected to a series of cooling and hydration steps in which the NO is completely oxidized to NO2, which in turn combines with water (some of which is present in the product gas, the rest of which is added) to form a 55 wt% aqueous nitric acid solution. The NO formed in the latter reaction is reoxidized and the added NO2 is hydrated to form still more HNO3. The product gas from the process may be taken to contain only N2 and O2. A simplified flowchart of the process follows.
(a) Taking a basis of 100 mol of ammonia fed to the process, calculates (i) the volume (m3) of the ammonia vapor and of the air fed to the process, using the compressibility factor equation of state for the ammonia calculation; (ii) the moles and molar composition of the gas Leaving the converter, and (iii) the required feed of Liquid water (m3) to the cooling and hydration step.
(b) Scale up the results calculated in part (a) to a new basis of 1000 metric tons of 55% nitric acid solution produced.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





