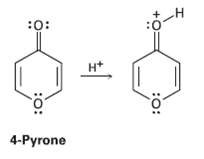
On reaction with acid, 4-pyrone is protonated on the carbonyl-group oxygen to give a stable cationic product.
Question:
On reaction with acid, 4-pyrone is protonated on the carbonyl-group oxygen to give a stable cationic product. Using resonance structures and the Hückel 4n + 2 rule, explain why the protonated product is sostable.
Transcribed Image Text:
:0: :0: H* 4-Pyrone :o: :o:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
666666 Protonation of 4pyrone gives structure A whic...View the full answer

Answered By

Fahmin Arakkal
Tutoring and Contributing expert question and answers to teachers and students.
Primarily oversees the Heat and Mass Transfer contents presented on websites and blogs.
Responsible for Creating, Editing, Updating all contents related Chemical Engineering in
latex language
4.40+
8+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give the structures of the carbonyl compound and the amine used to form the following imines. (a) (b) (c) (d) (e) (f) NH N=CHCH, N CH
-
Give the product of the reaction of pentanoic acid with each of the following reagents: (a) Sodium hydroxide (b) Sodium bicarbonate (c) Thionyl chloride (d) Phosphorus tribromide (e) Benzyl alcohol,...
-
Give an example of (a) A weak acid that contains oxygen atoms. (b) A weak acid that does not contain oxygen atoms. (c) A neutral molecule that acts as a Lewis acid. (d) A neutral molecule that acts...
-
Refer to the Journal of Experimental Psychology-Applied (June 2000) name-retrieval study, presented in Exercise. The data for the study are saved in the NAMEGAME2 file. a. Find a 99% confidence...
-
What place would efficient job design have in a company like BraunAbility? How could BraunAbility improve job efficiency in a way that is consistent with the company's emphasis on inclusiveness and...
-
Casey Hyunh is trying to value the stock of Resources Limited. To easily see how a change in one or more of her assumptions affects the estimated value of the stock, she is using a spreadsheet model....
-
For each of the following situations, calculate the \(t\)-statistic \((t)\) : a. \(\mathrm{X}^{-}=20.00 ; \mu=18 ; s \mathrm{X}^{-}=1.00\) b. \(X^{-}=20.00 ; \mu=13 ; s X^{-}=1.00\) c. \(X^{-}=12.00...
-
El Pico Company has two divisions: Maya and Aztec. During the year they had the following operating data: 1. Compute each division's contribution and segment margins, and the contribution each makes...
-
Find the vertical asymptotes, if any, and the values of x corresponding to holes, if any, of the graph of the rational function.. f(x)= x-6 X-9x+18
-
Write a symbolic microprogram routine for the ISZ (increment and skip if zero) instruction defined in Chap. 5 (Table 5-4). Use the microinstruction format of Sec. 7-3. Note that DR = 0 status...
-
Bextra, a COX-2 inhibitor used in the treatment of arthritis, contains an isoxazole ring. Why is the ringaromatic? Isoxazole ring H2N CH3 Bextra
-
Compound A, C8H10, yields three substitution products, C8H9Br, on reaction with Br2. Propose two possible structures for A. The 1H NMR spectrum of A shows a complex four-proton multiplet at 7.0 and...
-
An article in the Journal of Aircraft (1988) describes the computation of drag coefficients for the NASA 0012 airfoil. Different computational algorithms were used at with the following results (drag...
-
Research and identify a company that uses big data to improve business performance. Explain how big data has assisted this organization in a category of your choosing. This may include product...
-
Gary's Construction business employed 24 full time workers last year. This year, due to increased business opportunities, they had to hire an additional 8 full time workers. Determine the rate of...
-
Summary of the children's interests, the Bishop's mathematical activities, the 5Es, and the mathematical/science concepts evident in their play. Based on observation and summary of the children's...
-
While effective resourcing and talent management strategies can drive business success, discuss the key challenges that telecommunication company may face?
-
Reflect on one situation (either workplace or family/community settings) when you had to motivate someone to do something important and failed. 1. Why did that happen? Provide at least two specific...
-
Prove the second result of Theorem 4.11. That is, let \(\left\{X_{n}ight\}_{n=1}^{\infty}\) be a sequence of random variables that converge weakly to a random variable \(X\). Let...
-
When is the indirect pattern appropriate, and what are the benefits of using it?
-
Express the equilibrium constant for the chemical equation: CH3OH(g) CO(g) + 2 H(8)
-
(a) Circle the isoprene units in the following terpenes. (b) Classify each of these as a monoterpene, diterpene, etc. -farnese ne (from oil of citronella limonene -plnene zingiberene (from ol of...
-
Draw the structure of an example of each of the following types of lipids: (a) A saturated fat (b) A polyunsaturated oil (c) A wax (d) A soap (e) A detergent (f) A phospholipid (g) A prostaglandin...
-
Give the general classification of each compound. (a) glyceryl tripalmitate (b) (c) (d) (e) (f) CH3(CH210 CH2 Na CH3 (CH21C (CH216-CH3 tetradecyl octadecanoate CH3 CH HoC caryophyllene (from cloves)...
-
Techuxia Corporation worked on four jobs during October: Job A256, Job A257, Job A258, and Job A260. At the end of October, the job cost sheets for these jobs contained the following data: Beginning...
-
You are considering your retirement plans. You would like to buy a NICE RV and see the country. This will cost $281,000 You have $37,000 to open the account and you will deposit $685 at the end of...
-
Find functional dependencies. Date Slot StartTime End Time Class Subject 21/10/2018 1 7:00 8:30 SE1023 Database 21/10/2018 2 8:45 10:15 SE1022 Database 21/10/2018 3 10:30 12:00 SE1016 Database Date...

Study smarter with the SolutionInn App


