Propane gas enters a continuous adiabatic heat exchanger? at 40?C and 250 kPa and exits at 240?C
Question:
Propane gas enters a continuous adiabatic heat exchanger? at 40?C and 250 kPa and exits at 240?C superheated steam at 300?C and 5.0 bar enters the exchanger flowing counter currently to the propane and exits as a saturated liquid at the same pressure.
(a) Taking as a basis 100 mol of propane fed to the exchanger, draw and label a process flowchart. Include in your labeling the volume of propane fed (m3), the mass of steam fed (kg), and the volume of steam fed (m3).
(b) Calculate values of the labeled specific enthalpies in the following inlet?outlet enthalpy table for this process.
(c) Use an energy balance to calculate the required mass feed rate of the steam. Then calculate the volumetric feed ratio of the two streams (m3 steam fed/m3 propane fed). Assume ideal gas behavior for the propane but not the steam and recall that the exchanger is adiabatic.
(d) Calculate the heat transferred from the water to the propane (kJ/m3 propane fed). (Do an energy balance on either the water or the propane rather than on the entire heat exchanger.)
(e) Over a period of time, scale builds up on the heat transfer surface, resulting in a lower rate of heat transfer between the propane and the steam. What changes in the outlet streams would you expect to see as a result of the decreased heat transfer?
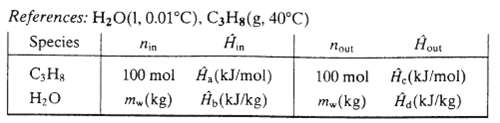
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





