A sample consisting of 1 mol of perfect gas atoms (for which CVm = 3/2 R) is
Question:
A sample consisting of 1 mol of perfect gas atoms (for which CV•m = 3/2 R) is taken through the cycle shown in Fig. 2.34.
(a) Determine the temperature at the points 1, 2, and 3.
(b) Calculate q, w, ∆U, and ∆H for each step and for the overall cycle. If a numerical answer cannot be obtained from the information given, then write in +, -, 0, or? As appropriate
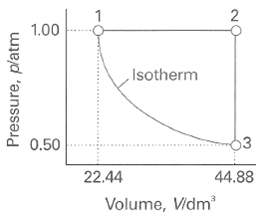
Transcribed Image Text:
1.00 ,Isotherm 13 0.50 22.44 44.88 Volume, Vidm' Pressure, platm
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (12 reviews)
The temperatures are readily obtained from the perfect gas equation T PV nR 100 atm x 224 dm 100 mol ...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physical Chemistry questions
-
Gas within a chamber passes through the cycle shown in Figure. Determine the energy transferred by the system as heat during process CA it the energy added as heat QAB during process AB is 20.0 J, no...
-
A monatomic ideal gas is taken around the cycle shown in Fig. 20.29 in the direction shown in the figure. The path for process c → a is a straight line in the pV-diagram. (a) Calculate Q, W, and...
-
A sample consisting of 2.00 mol of perfect gas molecules, for which CV, m= 5/2 R, initially at PI = 111 kPa and TI = 277 K, is heated reversibly to 356 K at constant volume. Calculate the final...
-
M1 is a way to measure... a) the level of bank reserves b) a country's money supply c) the level of savings in a country d) a country's economic potential
-
Discuss how the Frank-Starling law of the heart helps to explain the influence of venous return on stroke volume.
-
White Dove Company began operations in 2012 by selling a single product. Data on purchases and sales for the year were as follows: On January 4, 2013, the president of the company, Joel McLees, asked...
-
The components of a velocity field are given by \(u=x+y\), \(v=x y^{3}+16\), and \(w=0\). Determine the location of any stagnation points \((\mathbf{V}=0)\) in the flow field.
-
1. Using the Buyers Perception of Value presented in Figure 6.1, discuss the value provided by the MDVIP business model. Do you believe that MDVIP offers a good value to patients? 2. Based on the 10...
-
Discuss the implications of Quality by Design (QbD) in bioprocessing for regulatory compliance and process robustness. How do risk assessment and design of experiments (DoE) play a role in ensuring...
-
A group of student investors in Hong Kong opened Campus Laundromat Inc. on September 1, 2014. During the first month of operations, the following transactions occurred. Sept. 1 Shareholders invested...
-
Given that = 1.11 K atm-I for carbon dioxide, calculate the value of its isothermal Joule- Thomson coefficient. Calculate the energy that must be supplied as heat to maintain constant temperature...
-
A sample consisting of2.0 mol CO2 occupies a fixed volume of 15.0 dm3 at 300 K. When it is supplied with 2.35 kJ of energy as heat its temperature increases to 341 K. Assume that CO2, is described by...
-
Find the present and future values of an income stream of $12,000 a year for 20 years. The interest rate is 6%, compounded continuously.
-
Which of the following is correct about a Coverdell ESA? A. All earnings must be paid out at age 18. B. Contributions may only be made by a parent. C. Contributions to the ESA are tax-deductible. D....
-
Which of the following is true regarding Medicare benefits for a long-term care facility? I. Benefits are only provided for 20 days. II. Benefits are only provided for 100 days. III. No benefits are...
-
What type of investment risk is associated with an S&P index fund? A. Diversifiable risk. B. Nondiversifiable risk. C. Nonmarket risk. D. Nonsystematic risk.
-
Mr. and Mrs. Wilson want to establish QTPs for Dennis and their three other grandchildren, all of whom are under age11. What is the maximum amount they can contribute in one year without making a...
-
Alternative investments are obtained through the use of A. Commodities. B. Exchange traded funds. C. Hedge funds. D. Mutual funds.
-
What would be the result if a new state modified a decree where the decree state had continuing jurisdiction?
-
Which property determines whether a control is available to the user during run time? a. Available b. Enabled c. Unavailable d. Disabled
-
Understand how planning occurs in todays organizations.
-
Refer to the information in Exercise 6.15(b) and sketch the cooling curves for liquid mixtures in which x(B 2 H 6 ) is (a) 0.10, (b) 0.30, (c) 0.50, (d) 0.80, and (e) 0.95. Data in Exercise 6.15(b)...
-
Methane (melting point 91 K) and tetrafluoromethane (melting point 89 K) do not form solid solutions with each other, and as liquids they are only partially miscible. The upper critical temperature...
-
Show that two phases are in mechanical equilibrium only if their pressures are equal.
-
What is the worst case running time of the following sudo codes, in 0- notation? Suppose that all arithmetic operations (including simple multiplication) take a constant amount of time. Justify your...
-
4. Let G be a pseudorandom generator with expansion factor (n) > 2n. In each of the following cases, say whether G' is necessarily a pseudorandom generator and explain why or why not. Here, "||...
-
Write the code for the del () method in the following doubly linked list class public class ObjDList { private Obj Node list; private Obj Node tail; public ObjDList() { list = null; tail = null; }...

Study smarter with the SolutionInn App


