A gas turbine combustion chamber receives air at 6 bar and (500 mathrm{~K}). It is fuelled using
Question:
A gas turbine combustion chamber receives air at 6 bar and \(500 \mathrm{~K}\). It is fuelled using octane \(\left(\mathrm{C}_{8} \mathrm{H}_{18}\right)\) at an equivalence ratio of 0.8 (i.e. weak), which burns at constant pressure. The amount of carbon dioxide and water in the products are \(9.993 \%\) and \(11.37 \%\) by volume respectively. Calculate the maximum temperature achieved after combustion and evaluate the degrees of dissociation for each of the following reactions.
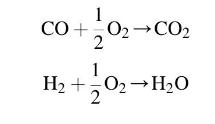
Assume the octane is in liquid form in the reactants and neglect its enthalpy; use an enthalpy of reaction of octane at \(300 \mathrm{~K}\) of \(-44820 \mathrm{~kJ} / \mathrm{kg}\).
\([0.0139 ; 0.00269 ; 2200 \mathrm{~K}]\)
Step by Step Answer:

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan





