A thermally isolated system at constant pressure consists of (10 mathrm{~kg}) of air at a temperature of
Question:
A thermally isolated system at constant pressure consists of \(10 \mathrm{~kg}\) of air at a temperature of \(1000 \mathrm{~K}\) and \(10 \mathrm{~kg}\) of water at \(300 \mathrm{~K}\), connected together by a heat engine. What would be the equilibrium temperature of the system if
1. the heat engine has a thermal efficiency of zero;
2. the heat engine is reversible?
Assume
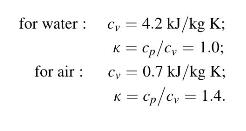
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan
Question Posted:





