The rate of formation of nitric oxide (NO) is controlled by the three reversible chemical reactions Use
Question:
The rate of formation of nitric oxide (NO) is controlled by the three reversible chemical reactions
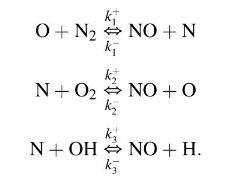
Use the steady state approximation for the nitrogen atom concentration and the assumption of partial equilibrium for the reactions governing the concentrations of \(\mathrm{O}, \mathrm{O}_{2}, \mathrm{H}\) and \(\mathrm{OH}\) show that
\[\beta=\frac{\delta R+\alpha}{\alpha R+1}\]
where \(\beta=[\mathrm{N}] /[\mathrm{N}]_{\mathrm{e}}, \delta=\left[\mathrm{N}_{2}\right] /\left[\mathrm{N}_{2}\right]_{\mathrm{e}}, \alpha=[\mathrm{NO}] /[\mathrm{NO}]_{\mathrm{e}}, R=R_{1} /\left(R_{2}+R_{3}\right)\), and \(R_{j}\) is the equilibrium reaction rate of reaction \(j\), and [ ] denotes the molar concentration, and [ \(]_{\mathrm{e}}\) is the equilibrium molar concentration. Derive an expression for \(\mathrm{d}[\mathrm{NO}] / \mathrm{d} t\) in terms of \(R_{1}, \beta, \alpha\) and \(\delta\).
At a particular stage in the formation of nitric oxide the values of \(R\) and \(\alpha\) are 0.26 and 0.1 re spectively. Why is \(\delta=1\) likely to be a good approximation in this case? What is the error if the rate of formation of \(\mathrm{NO}\) is evaluated from the larger approximation \(\mathrm{d}[\mathrm{NO}] / \mathrm{d} t=2 R_{1}\), rather than the equation derived in this question?
[3.64\%]
Step by Step Answer:

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan





