Consider a gas-liquid separation in which acetone in air is absorbed into water to purify the exhaust
Question:
Consider a gas-liquid separation in which acetone in air is absorbed into water to purify the exhaust air. The equilibrium is described by Raoult’s law, and the vapor pressure is given by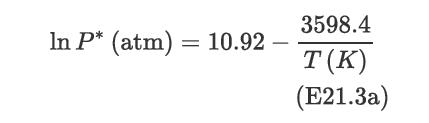
An absorber is to be designed to treat 100 kmol/h of air containing a mole fraction of 0.05 acetone and reduce it to a mole fraction of 0.0001. Pure water is used as the solvent at a rate of 50 kmol/h.
1. At a temperature of 35°C and a pressure of 1.25 atm, how many equilibrium stages are needed in a tray tower?
2. At the same conditions as in Part (a), how many transfer units are needed in a packed tower?
3. For the tray tower, suggest another set of conditions that would accomplish the desired separation. Any parameter may be changed other than the inlet gas conditions.
4. Suppose it is suggested that an existing tray tower with 7 stages be used for this separation. At the same temperature and pressure, what liquid flowrate is needed?
5. Suppose it is suggested that an existing packed tower with 15 transfer units be used with all other operating conditions unchanged. What outlet mole fraction is expected?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





