Consider the ammonia process in Problem 6.12. For the given conditions, the maximum single-pass conversion obtained in
Question:
Consider the ammonia process in Problem 6.12. For the given conditions, the maximum single-pass conversion obtained in the reactor is about 15%−20%. Explain how the temperature and pressure should be adjusted to increase this conversion of this equilibrium limited reaction and the penalties for making these changes.
Problem 6.12
The production of ammonia (a key ingredient for fertilizer) using the Haber process takes place at temperatures of around 500°C and pressures of 250 atm using a porous iron catalyst according the following highly exothermic synthesis reaction: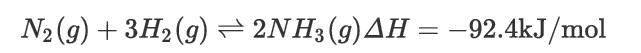
Give possible reasons for the high temperature and pressure used for this reaction.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





