Consider the same problem as Problem 13.27, but now develop a nonequilibrium-stage model with 20 theoretical stages.
Question:
Consider the same problem as Problem 13.27, but now develop a nonequilibrium-stage model with 20 theoretical stages. The first two reactions shown in Problem 13.27, even though reversible, are kinetically limited, while the rest of the reactions can be considered to be fast and at equilibrium. Consider using single-pass valve trays with the appropriate diameter (which can be calculated by the process simulator —assume 80% of flooding) and 152 valves/m of active area. Consider a tray spacing of 0.6096 m and a weir height of 46.55 mm. Fix the top pressure at 1.6 atm and calculate the pressure drop through the entire column. Depending on the process simulator available to you, consider appropriate transport models and account for the mass transfer resistance in both the liquid and vapor films. Since the related ionic reactions are very fast, consider that the ionic reactions also take place in the liquid film. Compare the results for Streams 3 and 4 with the results from Problem 13.27.
Problem 13.27
Figure P13.27 is the absorber in an acid-gas removal (AGR) plant.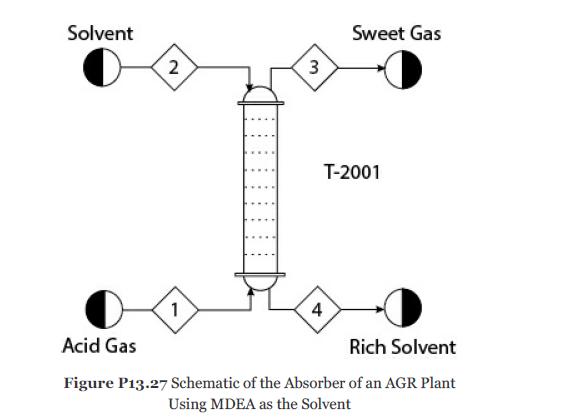
The solvent, Stream 2, in this plant is a chemical solvent, methyl diethanolamine (MDEA). It is fed to the top of the column. The acid gas, Stream 1, is fed to the bottom of the column. The conditions of Streams 1 and 2 are given in Table P13.27. The column has 20 theoretical stages. Simulate an equilibrium-stage model of this column considering the important ionic reactions and a suitable thermodynamic model for this electrolyte system. The column top pressure is 1.6 atm. Consider the following reactions:
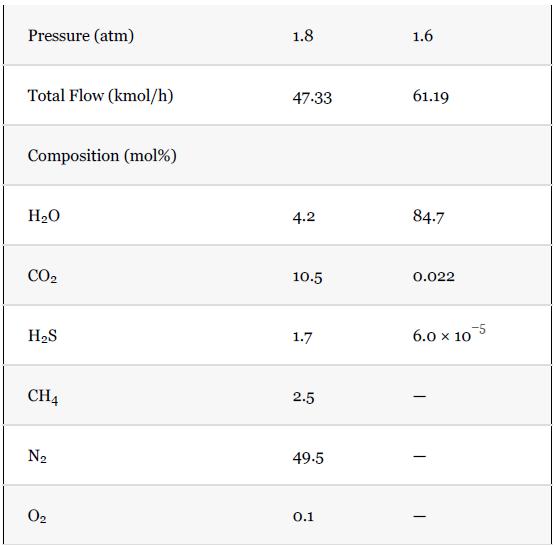
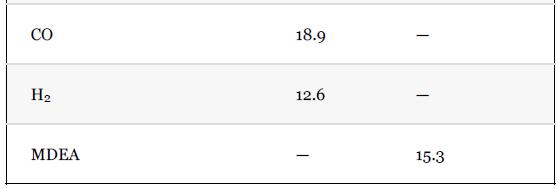
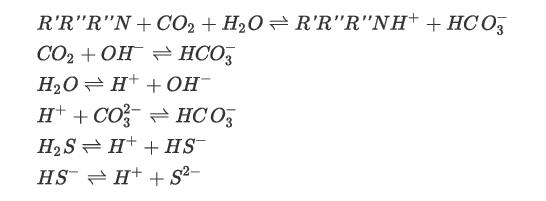
What are the compositions and temperatures of Stream 3 and Stream 4?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





