Develop the model of a sour-water stripper (SWS) as shown in Figure E13.7(a). Consider the following ionic
Question:
Develop the model of a sour-water stripper (SWS) as shown in Figure E13.7(a). Consider the following ionic reactions: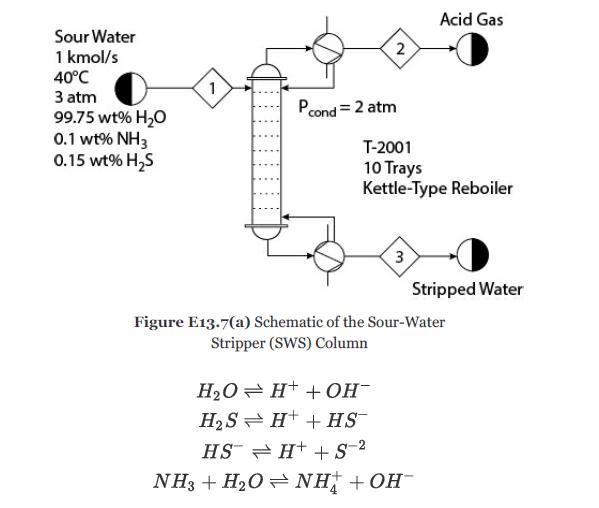
For this system do the following:
1. Simulate both an equilibrium-stage and a nonequilibrium-stage model and compare the key results such as reflux ratio (RR), reboiler duty, and so on.
2. For comparison, also simulate an equilibrium-stage and a nonequilibrium-stage model without considering the electrolyte chemistry, that is, without the ionic reactions.
3. Develop another nonequilibrium-stage model without considering the fourth reaction.
The desired specification for the separation is that the bottom product should not have more than 30 ppmw NH3 and 10 ppmw H2S and the top product should have 25% (mole basis) acid gases (H2S and CO2).
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





