For the reactor given in Example 22.9, determine the pressure drop across the reactor. Assume that the
Question:
For the reactor given in Example 22.9, determine the pressure drop across the reactor. Assume that the gas has the properties of air at the inlet conditions.
Example 22.9
The synthesis of phthalic anhydride from o-xylene is considered. The following parameters and pseudo-firstorder reaction rate may be used for an approximate preliminary design of a shell-and-tube reactor.![]()
The autoignition temperature of o-xylene = 465°C and lower explosion limit (LEL) = 0.9% at room temperature.
Data: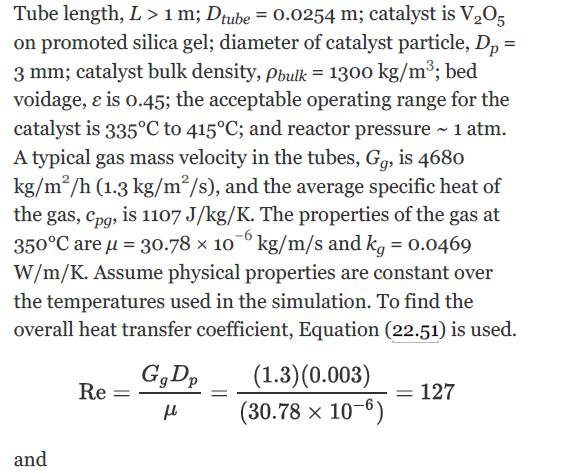
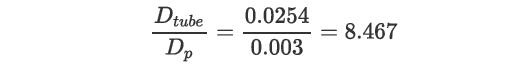
which are in the correct range to use Equation (22.51),![hw,eff Dtube kg (2.03) (127) 0.8 exp[-8.467] = 48.17 (48.17) (0.0469) = 88.9 W/m/K (0.0254) andUhw,eff = 88.9](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/3/9/614654b63fe304681699439613312.jpg)
The reaction rate can be approximated by a pseudo-firstorder equation: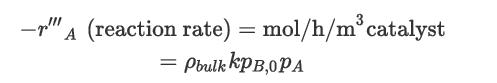
where Component A is o-xylene, k = 4.122 × 108e−13,636/T[K] mol/h/bar2/kg (catalyst), and Component B is oxygen with an initial partial pressure, pB,o = 0.21 bar.
The average heat of reaction for this temperature range −ΔHr is 1150 kJ/mol.
For illustration purposes, it is assumed that cooling takes place by heat exchange with a cooling medium flowing through the shell side at 335°C that vaporizes at constant pressure and temperature. With this assumption, the shell-side temperature, Tcool, is fixed at 335°C.
Determine the temperature profiles in the tube for partial pressures of o-xylene in the feed ranging from 0.01 to 0.02. Assume that the effect of changing pressure is small; that is, assume constant pressure conditions and a process gas inlet temperature of 335°C.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





