For the setup in Problem 22.25, do the following: 1. Determine the change in IPA conversion if
Question:
For the setup in Problem 22.25, do the following:
1. Determine the change in IPA conversion if the flowrate to the reactor increases by 25% but the utility flow does not change.
2. Determine the change in utility flowrate that must accommodate the change in feed gas rate in order to maintain the same conversion of IPA as in Problem 22.25.
Problem 22.25
Consider the dehydration of isopropyl alcohol (IPA) to yield acetone and hydrogen: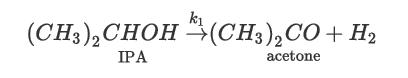
This reaction is endothermic, with a heat of reaction of 57.2 kJ/mol. The reaction is kinetically controlled and occurs in the vapor phase over a catalyst. The reaction kinetics for this reaction are first order with respect to the concentration of alcohol and can be estimated from the following equation:![-Ea RT [K] -TIPA = ko exp |- R CIPA kmol/m (catalyst)/s](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/4/4/2/186654b6e0a3c16a1699442184507.jpg)
where Ea = 72.38 MJ/kmol, ko = 1.931×105 m3 (gas)/m3 (reactor)/s, and cIPA has units of kmol/m3 (gas).
The feed to the reactor is 87 wt% IPA in water at 240°C and 2 bar. Spherical catalyst particles (5 mm in diameter) are placed in 50 mm ID tubes of length 6 m. The heat transfer coefficient on the tube side of the reactor is limiting. A heat transfer medium (HTM) is available as part of a heating loop that supplies the HTM to the reactor shell at 400°C.
Properties of the heat transfer fluid are
Properties of the process gas are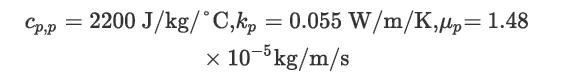
Properties of the catalyst are![]()
1. Determine the conversion of alcohol to acetone assuming that the HTM flows cocurrently with the process gas. Assume that the HTM flows at a rate of 200,000 kg/h, the inlet flowrate to the reactor is 8,000 kg/h of IPA and water, and the reactor has a total of 1600 tubes arranged on a square pitch with the tube centers 75 mm apart.
2. Sketch the temperature concentration profiles in a single reactor tube.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting




