In the process in Example 21.1, the vapor stream contains too much benzene, a valuable reactant. The
Question:
In the process in Example 21.1, the vapor stream contains too much benzene, a valuable reactant. The feed flowrate of benzene is 102 kmol/h, while the flowrate of benzene in the liquid phase is (192.14 kmol/h)(0.515) = 98.95 kmol/h, which is about 97% recovery of benzene in the liquid and a loss of about 3 kmol/h of benzene, which has a value of several million dollars/year. Under what operating conditions would 99% recovery of benzene in the liquid stream be possible?
Example 21.1
In the production of cumene from propylene and benzene, the feed propylene contains propane, which does not react with the benzene. The reaction occurs in the vapor phase. The separation section of the process starts with a partial condensation of the unreacted benzene (present in excess to enhance selectivity) and cumene to remove the propane. For this example, it is assumed that all propylene in the feed reacts and that there are no unwanted by-products in the reactor effluent. The feed to the partial condenser contains 51 mol% benzene, 44 mol% cumene, and the remainder propane. Ideal behavior is assumed, and the Antoine coefficients are shown in Table E21.1 and are assumed to be valid over the temperature range of this problem.
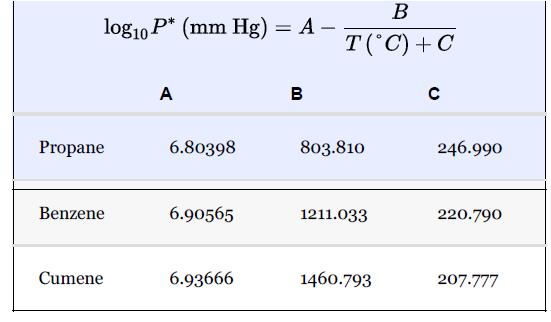
If a partial condensation of 200 kmol/h occurs at 90°C and 1.75 bar, what are the flowrates and mole fractions of the streams leaving the flash drum?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





