It is known that for a certain third-order, gas-phase reaction, the rate of reaction doubles when the
Question:
It is known that for a certain third-order, gas-phase reaction, the rate of reaction doubles when the temperature goes from 250°C to 270°C. The form of the rate equation is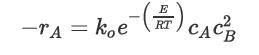
If the rate of reaction is 10 moles/m3/s at the base conditions, which are 20 atm pressure and 250°C, and an equal molar flow of A and B are in the feed (no inerts), answer the following questions:
1. Compared to the base case, by how much does the rate of reaction (at inlet conditions) change if the temperature is increased to 260°C?
2. Compared to the base case, by how much does the rate of reaction (at inlet conditions) change if the pressure is increased by 15%?
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





