p-Xylene is commercially separated from a mixture of xylenes in a crystallizer, because its freezing point is
Question:
p-Xylene is commercially separated from a mixture of xylenes in a crystallizer, because its freezing point is much higher than that of its other isomers. The feed is first cooled and then sent to a scraped-surface heat exchanger. Since the wall of this heat exchanger is at a very low temperature, the crystals are formed on the wall. These crystals are then removed by scraping with a spring-loaded blade. Two stages are used to increase the product purity. For modeling purposes, only one stage of this process will be considered in this example. This first stage will be modeled as a chiller, E-2001, followed by a crystallizer, CR-2001, as shown in Figure E13.8(a).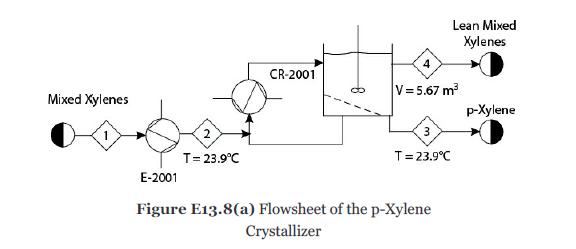
Conditions of Stream 1 in Figure E13.8(a) are as follows: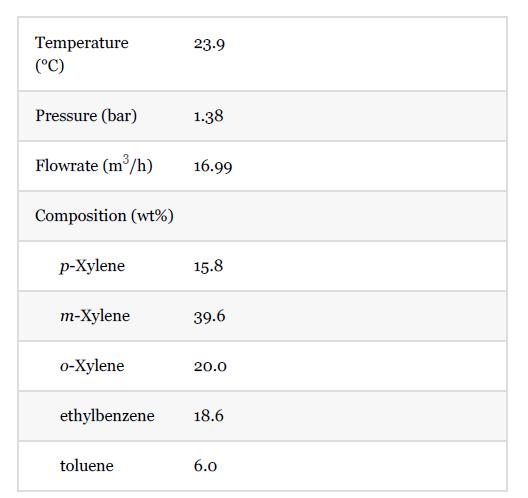
For simplicity, consider that no recirculation occurs in the crystallizer. The crystal growth rate is given by
Xs is the mole fraction of solute in the liquid, and Xs, eq is the mole fraction of solute in the liquid at crystallization temperature. The nucleation rate is given by![]()
Simulate this system and find out the mole fraction of pxylene in the solvent in Stream 4, the flowrate of solid pxylene leaving the crystallizer (flowrate of Stream 3), and the supersaturation ratio of Stream 4.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





