The residue curve map of Figure 12.9(a) shows that batch distillation of a mixture at the azeotropic
Question:
The residue curve map of Figure 12.9(a) shows that batch distillation of a mixture at the azeotropic composition for the system (A + B) with a small amount of C added will result in a very pure liquid C residue in the still. Is this a good separation unit choice to obtain “pure” C? Analyze the advantages and disadvantages.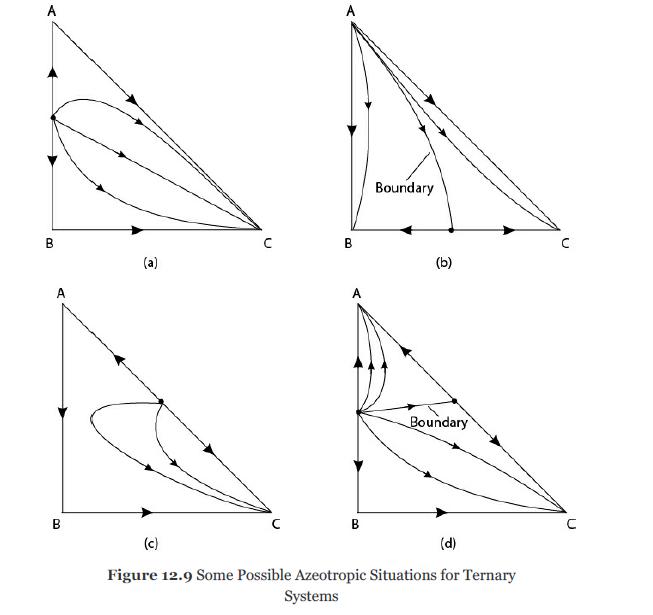
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting
Question Posted:





