There are two technically viable routes to the production of a hydrocarbon solvent, S, starting with feed
Question:
There are two technically viable routes to the production of a hydrocarbon solvent, S, starting with feed material A. Route 1 uses a disproportionation reaction, in which feed material A is converted to the desired solvent S and another solvent R, both of which are marketable products. Route 2 starts with the same chemical A but uses a hydrodealkylation reaction to produce the desired solvent. The reaction schemes for each process are shown below.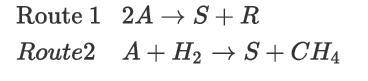
Assuming that pure A is fed to the process, the solvents S and R are separable by simple distillation, and both are much less volatile than either methane or hydrogen, sketch PFDs for Routes 1 and 2. Which process do you think will be more profitable? Explain your reasoning and assumptions.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting




