Calculate the pH of a buffer solution that contains 0.10 M acetic acid (Table 2.6) and 0.25
Question:
Calculate the pH of a buffer solution that contains 0.10 M acetic acid (Table 2.6) and 0.25 M sodium acetate.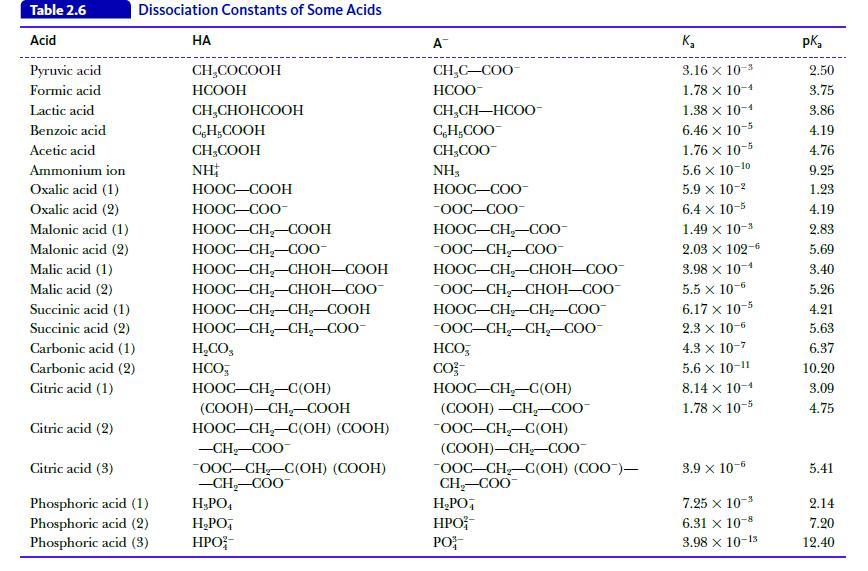
Transcribed Image Text:
Table 2.6 Acid Pyruvic acid Formic acid Lactic acid Benzoic acid Acetic acid Dissociation Constants of Some Acids Ammonium ion Oxalic acid (1) Oxalic acid (2) Malonic acid (1) Malonic acid (2) Malic acid (1) Malic acid (2) Succinic acid (1) Succinic acid (2) Carbonic acid (1) Carbonic acid (2) Citric acid (1) Citric acid (2) Citric acid (3) Phosphoric acid (1) Phosphoric acid (2) Phosphoric acid (3) HA CH₂COCOOH HCOOH CH₂CHOHCOOH CH₂COOH CH₂COOH NH HOOC–COOH HOOC—COO- HỌOC–CH,COOH HOOC–CH_–C00- HOOC–CH,CHOH—COOH HOOC–CH,–CHOH–COO- HOOC–CH–CH_–COOH HOOC–CH,CH,–COO- H,CO, HCO HOOC–CH,–C(OH) (COOH)-CH₂-COOH HOOC–CH,–C(OH) (COOH) -CH₂-COO™ OOC-CH₂-C(OH) (COOH) -CH₂-COO™ H₂PO4 H₂PO HPO A™ CH₂C-COO HCOO- CH,CH—HCOO- C, H,COO CH₂COO™ NH3 HỌỌC—COO -OOC-COO- HOOC–CH,–C0O- -OOC-CH₂-COO- HOOC–CH–CHOH–COO OOC-CH₂-CHOH-COO HOOC–CH_CH,COO -OOC-CH₂-CH₂-COO- HCO, CO²- HOOC–CH–C(OH) (COOH) -CH₂-COO™ OOC-CH₂-C(OH) (COOH)-CH₂-COO™ OOC-CH₂-C(OH) (COO)— CH₂-COO™ H₂PO4 HPO PO K₂ 3.16 x 10-3 1.78 x 10-4 1.38 x 10-4 6.46 x 10-5 1.76 x 10-5 5.6 x 10-10 5.9 x 10-² 6.4 x 10-5 1.49 × 10-³ 2.03 × 102-6 3.98 x 10-4 5.5 x 10-6 6.17 x 10-5 2.3 x 10-6 4.3 x 10-7 5.6 x 10-11 8.14 x 10-1 1.78 x 10-5 3.9 x 10-6 7.25 x 10-3 6.31 x 10-8 3.98 x 10-15 pka 2.50 3.75 3.86 4.19 4.76 9.25 1.23 4.19 2.83 5.69 3.40 5.26 4.21 5.63 6.37 10.20 3.09 4.75 5.41 2.14 7.20 12.40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Use the HendersonHassel Bal...View the full answer

Answered By

S Mwaura
A quality-driven writer with special technical skills and vast experience in various disciplines. A plagiarism-free paper and impeccable quality content are what I deliver. Timely delivery and originality are guaranteed. Kindly allow me to do any work for you and I guarantee you an A-worthy paper.
4.80+
27+ Reviews
73+ Question Solved
Related Book For 

Biochemistry
ISBN: 9781305961135
9th Edition
Authors: Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Question Posted:
Students also viewed these Sciences questions
-
First, write the code to create a single DataFrame object in a function called load_ticket_data(). This function should return the full dataframe and take no parameters (you can assume the ticket...
-
QUESTION 4 (25 MARKS: 45 MINUTES) < Daniaa Beauty Sdn. Bhd. is a business that sells skincare to customers. The business has extended its market segment to northern part of Malaysia. In addition to...
-
Calculate the pH of a buffer solution that is 0.050 M in benzoic acid (HC 7 H 5 O 2 ) and 0.150 M in sodium benzoate (NaC 7 H 5 O 2 ). For benzoic acid, Ka = 6.5 * 10 -5 .
-
Problem Set 3 b. zero. c. negative. d. smaller than the variance. 22. Growth factors for the population of Atlanta in the past five years have been 1, 2, 3, 4, and 5. The geometric mean is a. 15. b....
-
You are the manager of a monopoly. A typical consumers inverse demand function for your firms product is P = 100 20Q, and your cost function is C(Q) = 20Q. a. Determine the optimal two-part pricing...
-
If you were called in as a communication consultant by Burger Barn executives, what kind of information would you gather in making an assessment about the likely future of Creamy Creations? What...
-
Which domain of life do eukaryotes belong to?
-
The post-closing trial balance for Bugeja Co. is shown on page 349. The subsidiary ledgers contain the following information: (1) accounts receivable?? B. Cordelia $2,500, I. Togo $7,500, T. Dudley...
-
Identifying who and what enters the controlled areas is the purpose of access control procedures.
-
Calculate the pH of a buffer solution prepared by mixing 75 mL of 1.0 M lactic acid (see Table 2.6) and 25 mL of 1.0 M sodium lactate. Table 2.6 Acid Pyruvic acid Formic acid Lactic acid Benzoic acid...
-
List the criteria used to select a buffer for a biochemical reaction.
-
Visitors to a popular Internet site rated the newest gaming console on a scale of 1 to 5 stars. The following probability distribution is proposed based on over 1400 individual ratings. f(x)...
-
Show the result of inserting the values 1, 2, 3, 4, 5, and 6 (in that order) into the B+-tree of Figure 10.17. 101215 18 23 18 19 20 21 22 33 233031 33 45 47 48 48 50 52
-
The EI mass spectrum of lead(II) acetate shows four peak envelopes, each with an isotope pattern characteristic of Pb. The most intense peak in each envelope appears at m/z 326.0, 267.0, 224.0 and...
-
What does nonrecourse financing mean?
-
Why do lenders charge origination fees and loan discount fees?
-
To be cognitively flexible seems to require that the entrepreneur continually question himself or herself. Doesnt that create doubt that can be seen by employees and financiers such that success...
-
Shelleys Kennel provides boarding for dogs and cats. Shelley, the owner of the kennel, must make several business decisions soon. Write yes or no to indicate whether knowing the cost to board one...
-
Write a paper by answer the following question: Should Recycling Be Mandatory?
-
Why is it somewhat misleading to study biochemical pathways separately?
-
What is the Warburg effect? Why would cancer cells favor such inefficient metabolism?
-
To what extent can metabolic pathways be considered reversible? Why?
-
3. Find the limits of the following sequences. Justify your answers. = 2+ 2 + ... + 2 (a) an In times In(n+1)- Inn (b) bn = Inn In(n+1)
-
David and Robert form an equal partnership. David contributed $10,000 cash to the partnership and Robert contributed depreciable property with a fair market value of $10,000 and an adjusted basis of...
-
1 2. Consider the integral 1/2 + 4 + 20 dr. (a) Complete the square for the denominator of the integrand. Then rewrite the integral using this expres- sion. (b) How does this help us evaluate the...

Study smarter with the SolutionInn App


