Shown below are two cartoon views of the small globular protein StrepG in which an a helix
Question:
Shown below are two cartoon views of the small globular protein StrepG in which an a helix is packed against a four-strand β sheet. The sheet is made up of two “β-hairpins” (a b-hairpin is a “β-turn-β” structure). Refer to the images and answer the questions that follow:
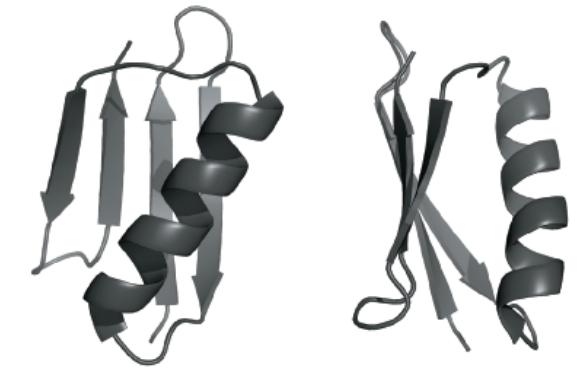
(a) Identify the locations of the N- and C-termini of StrepG.
(b) Indicate the orientation of the helical macrodipole, showing the (₰+) and (₰-) ends of the macrodipole.
(c) How many residues are in the helix?
(d) Do you predict that the a helix and β sheet are amphiphilic or not? Briefly explain.
(e) The following two peptides are part of the primary sequence of StrepG. Based on your answer to part (d), which one is more likely to correspond to the a helix? Which is most likely to be part of a β-hairpin? Explain your choice
Step by Step Answer:

Biochemistry Concepts And Connections
ISBN: 9780134641621
2nd Edition
Authors: Dean Appling, Spencer Anthony-Cahill, Christopher Mathews





