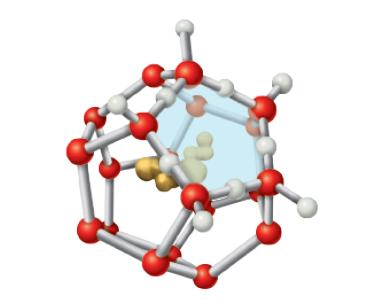
When a hydrophobic substance like a hydrocarbon is dissolved in water, a clathrate cage of ordered water
Question:
When a hydrophobic substance like a hydrocarbon is dissolved in water, a clathrate cage of ordered water molecules is formed about it (see Figure 2.14). If we consider only the effects on water, what do you expect the sign of ∆S to be for this process? Explain your answer.
Figure 2.14
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Biochemistry Concepts And Connections
ISBN: 9780134641621
2nd Edition
Authors: Dean Appling, Spencer Anthony-Cahill, Christopher Mathews
Question Posted:





