According to Maxwells Distribution Law, in a gas of molecular mass m, the speed v of a
Question:
According to Maxwell’s Distribution Law, in a gas of molecular mass m, the speed v of a molecule in a gas at temperature T (kelvins) is a random variable with density
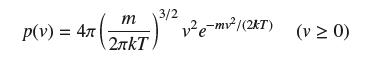
where k is Boltzmann’s constant. Show that the average molecular speed is equal to (8kT/πm)1/2. The average speed of oxygen molecules at room temperature is around 450 m/s.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





