The BeerLambert Law is used in spectroscopy to determine the molar absorptivity > 0 or the
Question:
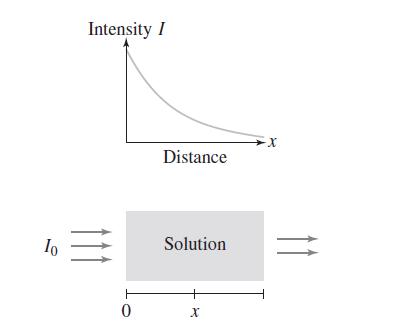
The Beer–Lambert Law is used in spectroscopy to determine the molar absorptivity α > 0 or the concentration c > 0 of a compound dissolved in a solution at low concentrations (Figure 1). The law states that the intensity I of light as it passes through the solution satisfies ln(I0/I) = αcx, where I0 is the initial intensity and x is the distance traveled by the light. Show that I decays exponentially as a function of x.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





