The pressure P, volume V, and temperature T of a van der Waals gas with n molecules
Question:
The pressure P, volume V, and temperature T of a van der Waals gas with n molecules (n constant) are related by the equation
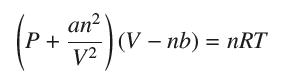
where a, b, and R are constant. Calculate ∂P/∂T and ∂V/∂P.
Transcribed Image Text:
(P + 1 ) (V V2 (V-nb) = nRT
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
By Eq 7 Let F be the following function We compute the parti...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
In physical chemistry, it is shown that the pressure P of a gas is related to the volume V and temperature T by van der Waals equation: where a, b, n, and R are constants. The critical temperature T...
-
Lets look at another way to compute phase diagrams from an equation of state without using departure functions but still requiring a numerical solution. The van der Waals equation of state can be...
-
North American Badgers (Taxidea taxus) occur throughout the western United States and Great Plains of North America, with the geographic range extending east to central Ohio (Messick, 1987; Whitaker...
-
A sedimentation process is to be used to separate pulverized coal from slate. A suspension of finely divided particles of galena (lead sulfide SG = 7.44) in water is prepared. The overall specific...
-
On what factors does the periodicity of the Periodic Table depend? Consider the exclusion principle, quantization of angular momentum, spin, and any others you can think of.
-
Determine the slope at the end \(B\) of the A-36 steel beam. \(I=80\left(10^{6} ight) \mathrm{mm}^{4}\). A 8 m 6 kNm
-
Santa Cruz Company has a tax rate of 20 percent on taxable income. It is considering a capital project that will make the following annual contribution to operating income: 1. Determine the net cash...
-
In the figure an electron (e) is to be released from rest on the central axis of a uniformly charged disk of radius R. The surface charge density on the disk is +4.27 C/m. What is the magnitude of...
-
When x, y, and z are related by an equation F(x, y, z) = 0, we sometimes write (z/x) y in place of z/x to indicate that in the differentiation, z is treated as a function of x with y held constant...
-
For all x > 0, there is a unique value y = r(x) that solves the equation y 3 + 4xy = 16. (a) Show that dy/dx = 4y/(3y + 4x). (b) Let g(x) = f(x, r(x)), where f(x, y) is a function satisfying...
-
A bond currently sells for $1,050, which gives it a YTM of 6%. Suppose that if the yield increases by 25 basis points, the price of the bond falls to $1,025. What is the duration of this bond?
-
Find the eigenvalues, eigenvectors, AM, and GM of each eigenvalue and decide whether the matrix is defective or not. Then, transform the matrix into either a diagonal or a Jordan matrix, whichever is...
-
Find the eigenvalues, eigenvectors, AM, and GM of each eigenvalue and decide whether the matrix is defective or not. Then, transform the matrix into either a diagonal or a Jordan matrix, whichever is...
-
1. Your instructor will divide the class into teams and assign each team the task of investigating the start-up of one of the following businesses: a. Submarine sandwich shop b. Day care service c....
-
The two insulated, current-carrying wires in Figure P28.7 cross at right angles, and each carries a current \(I\). The locations labeled 1-4 are all in the plane defined by the wires, with each...
-
Given \[\mathbf{A}=\left[\begin{array}{ccc}0 & 1 & 0 \\0 & 0 & 1 \\-2 & -1 & -2\end{array} ight], \quad\mathbf{C}=\left[\begin{array}{lll}2 & 0 & \frac{1}{3}\end{array} ight]\]find the rank...
-
Winans Company uses the lower-of-cost-or-market method, on an individual-item basis, in pricing its inventory items. The inventory at December 31, 2013, included product X. Relevant per-unit data for...
-
Give the products of the following reaction, where T is tritium: dioldehydrase Ad- CH CH3C-COH CoIII) coenzyme B12
-
According to the Girl Scouts of America, in March 2006, 9% of all Girl Scout cookies sold are shortbread/trefoils. If a box of Girl Scout cookies is selected at random, what is the probability that...
-
A golf ball is selected at random from a container. If the container has 9 white balls, 8 green balls, and 3 orange balls, find the probability of each event. The golf ball is white or green.
-
A golf ball is selected at random from a container. If the container has 9 white balls, 8 green balls, and 3 orange balls, find the probability of each event. The golf ball is white or orange.
-
. Resilience in African American single-parent households: Perceptions of predictors for academic success httos://www.academia.edu/downloa d/84373715/ee4laf12a30ecde9d7edb 479027b993ea759pdf...
-
This is for my Sociology 340: Juvenile Delinquency Discussion Post: The U.S. Supreme Court has determined which constitutional rights are afforded to juveniles, including how the Fifth Amendment and...
-
ESSAY-4-5 pages Upward economic mobility is difficult to attain in this country. You implicitly know this; that's why you've come to college. But just being in college isn't enough. How can you...

Study smarter with the SolutionInn App


