a. The first law of thermodynamics can be represented by the expression: U = q + W.
Question:
a. The first law of thermodynamics can be represented by the expression: ΔU = q + W. State what is meant by all the symbols in this expression.
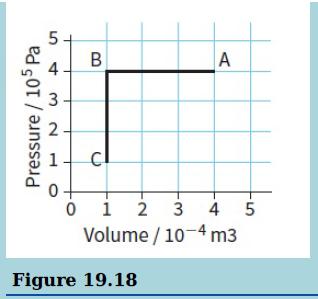
b. Figure 19.18 shows a fixed mass of gas that undergoes a change from A to B and then to C.
i. During the change from A to B, 220 J of thermal energy (heat) is removed from the gas. Calculate the change in the internal energy of the gas.
ii. During the change from B to C, the internal energy of the gas decreases by 300 J. Using the first law of thermodynamics explain how this change can occur.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:





