Compound B has the composition 62.1% carbon, 10.3% hydrogen and 27.6% oxygen. Its mass and 1H NMR
Question:
Compound B has the composition 62.1% carbon, 10.3% hydrogen and 27.6% oxygen. Its mass and 1H NMR spectra are shown below.
a.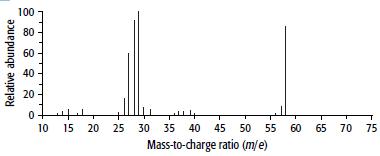
b.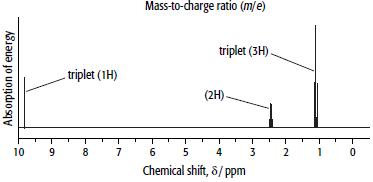
a. Calculate the empirical formula of B.
b. From the mass spectrum, find the molecular mass of B and hence its molecular formula.
c. i. Draw displayed formulae for the possible isomers of B which contain a carbonyl group.
ii. Use the 1H NMR spectrum of B to decide which isomer is B.
iii. Explain your reasoning.
d. Explain what caused the peak at δ = 1.1 ppm and why it is split into a triplet in the 1H NMR spectrum of B.
e. Predict the number of signal lines present on the carbon-13 NMR spectrum of:
i. Compound B, stating the origin of each line
ii. The isomer of compound B identified in your answer to c part i, stating the origin of each line.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





