Copper forms complexes with chloride ions and with ammonia. log 10 K stab for aqueous Cu 2+
Question:
Copper forms complexes with chloride ions and with ammonia.
log10 Kstab for aqueous Cu2+ ions with Cl– as a ligand is 2.80.
log10 Kstab for aqueous Cu2+ ions with NH3 as a ligand is 4.25.
a. A few drops of hydrochloric acid are added to blue aqueous copper(II) sulfate.
i. Copy and complete the equation for this reaction.
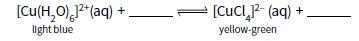
ii. Describe and explain what happens when excess water is then added to the reaction mixture in part i.
iii. Explain what happens in terms of ligand exchange, when concentrated aqueous ammonia is added to the reaction mixture from part ii.
b. Aqueous copper ions form a complex with 1,2-diaminoethane, NH2CH2CH2CH2NH2. They also form a complex with 1,3-diaminopropane NH2CH2CH2CH2NH2.
log10 Kstab for aqueous Cu2+ ions with 1,2-diaminoethane as a ligand is 20.3
log10 Kstab for aqueous Cu2+ ions with 1,3-diaminopropane as a ligand is 17.7
i. What type of ligands are 1,2-diaminoethane and 1,3-diaminopropane? Explain your answer.
ii. Which one of these ligands forms a more stable complex? Explain your answer.
iii. 1,2-diaminoethane can be abbreviated as ‘en’.
A complex of nickel ions, Ni2+, with 1,2-diaminoethane is octahedral in shape with a co-ordination number of 6. Draw the structures of the two stereoisomers of this complex.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





