This question is about two transition metals, hafnium (Hf) and zirconium (Zr). a. Hafnium forms a peroxide
Question:
This question is about two transition metals, hafnium (Hf) and zirconium (Zr).
a. Hafnium forms a peroxide whose formula can be written as HfO3.2H2O. Use the Ar values below to calculate the relative molecular mass of hafnium peroxide.
(Ar values: Hf = 178.5, H = 1.0, O = 16.0)
b. A particular isotope of hafnium has 72 protons and a nucleon number of 180. Write the isotopic symbol for this isotope, showing this information.
c. The mass spectrum of zirconium is shown below.
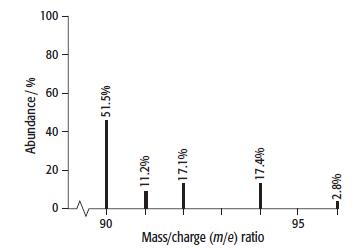
i. Use the information from this mass spectrum to calculate the relative atomic mass of zirconium. Give your answer to 3 significant figures.
ii. High-resolution mass spectra show accurate relative isotopic masses. What do you understand by the term relative isotopic mass?
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





