Zirconium, Zr, and hafnium, Hf, are metals. An isotope of zirconium has 40 protons and 91 nucleons.
Question:
Zirconium, Zr, and hafnium, Hf, are metals. An isotope of zirconium has 40 protons and 91 nucleons.
a. i. Write the isotopic symbol for this isotope of zirconium.
ii. How many neutrons are present in one atom of this isotope?
b. Hafnium ions, 180 72 Hf 2+, are produced in a mass spectrometer. How many electrons are present in one of these hafnium ions?
c. The subatomic particles present in zirconium and hafnium are electrons, neutrons and protons. A beam of protons is fired into an electric field produced by two charged plates, as shown in the diagram.
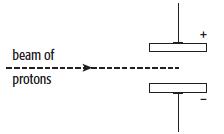
i. Describe how the beam of protons behaves when it passes through the gap between the charged plates. Explain your answer.
ii. Describe and explain what happens when a beam of neutrons passes through the gap between the charged plates.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris




