A fluid obeys the equation of state a. For what values of the constants B and C
Question:
A fluid obeys the equation of state
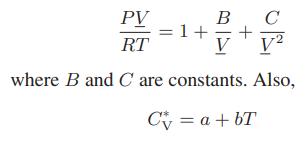
a. For what values of the constants B and C will this fluid undergo a vapor-liquid phase transition?
b. What is the molar internal energy change if this fluid is heated at a constant pressure P from T1 to T2, and how does this compare with the molar internal energy change for an ideal gas with the same ideal gas heat capacity undergoing the same change?
c. Develop an expression relating the differential change in temperature accompanying a differential change in volume for a reversible adiabatic expansion of this fluid. What would be the analogous expression if the fluid were an ideal gas?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





